Download this article in PDF format.
Engineers like to be involved. Even when up to our collective clavicles in code, we’re most comfortable when we can provide input on a project through all stages: from the initial concept; through the design and development stage; on to the testing of prototypes; and finally following the project right up to final manufacturing. These attributes mean engineers are well-suited to act with speed and create the critical equipment that hospitals need to combat the COVID-19 pandemic.
With the rise of COVID-19 cases throughout the United States, one of the biggest concerns is the shortage of ventilators for patients. The mechanical ventilators used in critical-care settings are complex, microprocessor‐driven devices designed to support a wide range of medical conditions. And they’re costly, so large‐scale stockpiling of such devices has proven to be financially impractical.
To address the coronavirus outbreak, teams of researchers are looking to design and manufacture new breathing devices that could be made rapidly by non-specialist companies. The core challenge is how to design and manufacture creative solutions in volume in an extremely short space of time.
Also on the possibly problematic side of the ledger is regulatory approval from the Food and Drug Administration (FDA). Happily, the FDA is operating on the basis of a U.S. Dept. of Health and Human Services (HHS) determination that circumstances exist to justify the authorization of emergency use of previously unapproved ventilators, anesthesia gas machines modified for use as ventilators, and positive-pressure breathing devices modified for use as ventilators during the COVID-19 pandemic.
Over the past few weeks, engineers have shown they’re prepared to lead the charge, helping the world respond to this crisis. It’s heartening to report that the response from engineers has been unprecedented, and many efforts to address the impact of COVID-19 are underway.
Sponsored Resources:
- AC/DC Desktop Adapter 24V 221W
- AC/DC Converter 24V 336W
- High Current - High Density Mini-Fit Jr. Series Product Family
But before we get to specifics, some background is in order. When a normal, healthy person breathes, he/she creates a negative pressure inside the lung by first expanding the chest wall and contracting the diaphragm. When the pressure inside the lung is lower than ambient, air flows into the lung. During expiration, air is exhaled passively, reducing the volume of the lung.
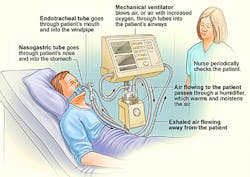
Ventilators play a critical role in the management of patients with severe respiratory illness, such as COVID-19, who require assistance because they cannot breathe effectively. These devices support breathing by getting oxygen into the lungs and removing carbon dioxide (Fig. 1). The oxygen can be controlled through a monitor. The ventilator is connected to the patient through a tube that is placed into the mouth or nose.
Modern ventilators are electronically controlled by a small embedded MPU. By placing a patient on a ventilator, the patient’s lungs are permitted to rest and recover while the ventilator performs the functions of supplying oxygen and simulating the actions of breathing. Without ventilation support, some patients with severe respiratory disease might not survive. In particular, the goal of mechanical ventilation is to provide these supports while protecting the lung from further damage.
Using What’s Available
What follows are examples of how teams of medical professionals and engineers have come together to develop equipment that can be used in situations where clinics no longer have sufficient regular ventilation machines available.
A team at the University of Minnesota has designed a mechanical ventilator that’s inexpensive and made of easy-to-obtain materials. Relying on parts and engineering input from electronics distributor Digi-Key, University of Minnesota anesthesiology fellow Stephen Richardson led a team that created a prototype for a low-cost ventilator—now called the Coventor—which has been tested successfully (Fig. 2).
Digi-Key’s Randall Restle told Electronic Design that he received a call from Richardson for help in determining how to make a ventilator work based on the idea of motorized Ambu bags. These resuscitation bags are used in first aid and are available in large quantities at low cost.
The motorized bags consist of a mask that’s pressed onto the face and a bag that’s compressed by hand by a medical professional or emergency technician at regular intervals to provide breaths to a patient in situations like cardiac arrest, until an intervention such as a ventilator becomes available. A tube is inserted into the patient’s airway, as with a hospital ventilator, but then the pumping of air into the lungs is done by squeezing and releasing the flexible pouch.
While Ambu bags are generally compressed by hand at regular intervals for ventilation, the team is developing mechanical devices that compress the bags periodically. Digi-Key worked with the team to determine the encoders, cables, and connectors needed and sent them overnight so that they received it the next day.
The proposed system consists of a frame and mechanical actuator that will stabilize and compress a commercially available ambulatory ventilation bag connected to the patient’s endotracheal tube and external compressed oxygen, or if oxygen isn’t available, ambient air. The Coventor is compact—about the size of a cereal box—and the frame can be metal-stamped or 3D-printed.
Version 2 of the prototype kept a pig alive. In version 3 the team, has improved the mechanical components for robustness. Specifically, according to Aaron Tucker, Lab Supervisor and Technical Development Coordinator at the University’s Earl E. Bakken Medical Devices Center, “We improved the housing to provide protection, the sliding elements to reduce friction, and the elements interacting with the ventilator bag to improve its standalone holding capability.”
Ventilators usually cost between $3,000 and $13,000. The team estimates that this new design can be built for around $1,500, and they hope the final version can be built for $1,200.
Constructed for rapid deployment, the Coventor uses off-the-shelf components such as Mean Well’s GSM220A24-R7B 220-W ac-dc medical adapter and MSP-300-24 24-V, 336-W ac-dc converter. It also employs the Molex Mini-Fit Jr. series of connectors, designed for high-current/high-density applications and appropriate for both power and signal applications as they can carry currents of up to 9.0 A per circuit and maintain a 10-mΩ contact resistance.
Digi-Key’s Restle notes that it may be possible for the ventilator to work using a windshield-wiper motor, but much engineering work still remains. Right now, he said, “They’re using an overkill motor for the proof of concept.” Added UMN’s Tucker: “As with any motor-related project, we are working on finding a motor which has a strong supply chain and meets our torque and speed requirements. We have been in collaboration with several companies to help us meet this goal.”
From BiPAP to Ventilator
In New York, a Northwell Health physician, a respiratory therapist, and a 3D-printing bioengineer successfully designed a way to turn the more common bi-level positive airway pressure (BiPAP) machine into a functional invasive mechanical ventilator, through a 3D-printed adapter they designed to aid in the conversion (Fig. 3).
BiPAP is one type of PAP, or positive airway pressure, non-invasive machine that’s commonly used to maintain a consistent breathing pattern at night. It’s also utilized during symptom flare-ups in people with sleep apnea, congestive heart failure, or chronic obstructive pulmonary disease (COPD), a chronic inflammatory lung disease.
PAP blower components are fairly abundant. In many of these devices, the blower is a simple BLDC motor that can be driven with an electronics speed controller (ESC) similar to that used by flying drones.
At Northwell, a team led by Hugh Cassiere, MD, medical director for respiratory therapy services at North Shore University Hospital (NSUH) and Stanley John, NSUH’s director of respiratory therapy, developed a method to convert the non-invasive Philips Respironics V60 BiPAP machine into a pressure-controlled ventilator for both patients with and without COVID-19-induced lung disease.
The key component to converting the BiPAP machine is a small, plastic T-piece adapter. “We were able to imitate the design of the T-piece adapter and print the plastic-resin piece with our 3D printers,” said Todd Goldstein, PhD, director of 3D Design and Innovation at Northwell Health. “If the need arises, we would be able to print 150 adapters in 24-hours.”
Drs. Cassiere, Goldstein, and Stanley John successfully tested the conversion of the BiPAP machine using the standard, non-3D printed adapter for both COVID-19 and non-COVID-19 patients.
In addition to the T-piece adapter, modifications to the BiPAP machine included the addition of two high-efficiency particulate-air (HEPA) filters at both ends of the oxygen hose to alleviate fears of spreading the virus.
The Model A-E and the E-Vent
Ford is helping GE Healthcare double its production of ventilators licensed from Airon Corp. The GE/Airon Model A-E ventilator uses a design that operates on air pressure without the need for electricity, addressing the requirements of most COVID-19 patients (Fig. 4).
The ventilators will be produced “around the clock” with the aid of 500 paid volunteers working in three shifts, with the goal of producing 50,000 of the vitally needed units within 100 days and up to 30,000 a month thereafter as needed. Ford and GE Healthcare also announced a separate effort to produce a simplified design of GE Healthcare's existing ventilator with Ford providing technical and production expertise.
A Massachusetts Institute of Technology (MIT) solution called MIT E-Vent (for emergency ventilator) is an open-source, low-cost ventilator design that expands on a project done in 2010 by students in MIT’s class 2.75 (Medical Device Design). However, in 2010, it didn’t move past the prototype stage.
In the original project, a tube was inserted into the patient’s airway, as with a hospital ventilator, but then the pumping of air into the lungs is done by squeezing and releasing the flexible pouch. The innovation begun by the earlier MIT class is now being rapidly refined and tested by the new team. They devised a mechanical system to do the squeezing and releasing of the Ambu bag (Fig. 5), since this isn’t something that a person could be expected to do for any extended period (around 1 million cycles would be required to support a ventilated patient over a two-week period).
This device has a very simple design, but it uses a modern electronic control system. It employs wood, tape, plastic bags, a threaded tube, solenoid valves, magnetic switches, and a PLC.
E-Vent only pushes atmospheric air (with 21% oxygen). For other oxygenation ratios, professional equipment is needed, but the device is useful and valuable in emergency situations when there’s no alternative.
Elsewhere Around the World
A consortium of leading UK industrial, technology, and engineering businesses has come together to produce medical ventilators for the UK using specifications for a Rapidly Manufactured Ventilator System (RMVS) developed by clinicians and the Medicines and Healthcare products Regulatory Agency (MHRA) (Fig. 6). The consortium has received formal orders from the Government in excess of 10,000 units. The new design is based on existing technologies, which can be assembled from materials and parts in current production.
Engineers at University College London (UCL) and Mercedes-AMG High Performance Powertrains, together with clinicians at University College Hospital, are reverse-engineering a CPAP (Continuous Positive Airway Pressure) device that could be rapidly manufactured and delivered to Britain’s National Institute for Health (NHS) hospitals. The device they produced, called UCL-Ventura, has gained regulatory approval. The British Government has now placed an order for up to 10,000.
Also in Europe, a team of researchers from the Philipps University of Marburg and the University Hospital Gießen and Marburg (UKGM) developed two different concepts for simple ventilators. The first concept is based on the use of CPAP devices to treat sleep apnea, which are available in many private households. Arduino-compatible software is used to control the motor speed and breathing cycles, and handle user input.
The CPAP devices will be expanded so that they can be utilized for artificial respiration. They work by pushing an air-oxygen mix into the mouth and nose at a continuous pressure, keeping airways open and increasing the amount of oxygen entering the blood stream.
The first prototypes are already running. Since the modified CPAP devices aren’t as powerful as professional ventilators, they’re not suitable for the initial treatment of acute, severe COVID-19 cases. However, if patients have recovered sufficiently after a few days and require less intensive ventilation, the modified CPAP devices could be used for ventilation respiratory distress. Clinical ventilators would then be available for the next patient with acute problems.
Appliance-maker Dyson received an order from the UK government for 10,000 ventilators to support efforts by the country's National Health Service to treat coronavirus patients. The company is working on a new ventilator design called the CoVent (Fig. 7). This design is meant to be made quickly and at high volumes, and leverages Dyson’s existing “Digital Motor” design, as well as the company’s air purification products. The motor itself isn’t digital, but the power regulator to the motor is a digital part.
The Dyson V6 is a brushless dc motor that can spin at well over 100,000 rpm. Brushless motors are able to offer great amounts of torque per watt and reduce wear and tear. The CoVent can run on battery power if needed, as a portable or bed-mounted device. According to Dyson, the CoVent was designed in just 10 days and the ventilators are slated for availability this month.
We’ve Faced Crises Before
As we make our way through this unprecedented crisis, we need to take a step back and remember engineers have met big challenges before. Recall, for example, after an explosion crippled the Apollo 13 spacecraft, the three astronauts went into the Lunar Module for the flight home and in doing so overloaded the carbon-dioxide scrubbers. The command module had scrubbers, but they were different shapes and sizes. Using only equipment available to the astronauts, NASA engineers in Houston were able to fashion a workaround that used the command-module scrubbers in the Lunar Module.
In preparation for the movie that told the story, the script writers Al Reinert and Bill Broyles interviewed Flight Controller Jerry Bostick. One of their questions was: "Weren't there times when everybody, or at least a few people, just panicked?" His answer was "No, when bad things happened, we just calmly laid out all the options, and failure was not one of them."
Then, as now.
Sponsored Resources:
- AC/DC Desktop Adapter 24V 221W
- AC/DC Converter 24V 336W
- High Current - High Density Mini-Fit Jr. Series Product Family
Related Resource: