What Can We Do with Pulse-Transit-Time Calculations from Wearables?
In the U.S., cardiovascular disease is the costliest. It’s been the leading cause of death for Americans since 1920. What’s more, related medical as well as indirect costs (such as from lost productivity) that totaled $555 billion in 2016 are expected to hit $1.1 trillion by 2035, according to the American Heart Association.1 Given this dire prognosis, it’s an opportune time to herald the emergence of wearable technologies that provide continuous, real-time monitoring of vital signs.
The future of health wearables will be about much more than step counting and the tracking of calories burned. Designed with sophisticated sensors, advanced algorithms, and powerful yet efficient processors, devices are already available that monitor parameters such as heart rate, blood-oxygen levels, sleep quality, and stress levels. Research and work is underway to bring devices to market for non-invasive detection or screening of such conditions as diabetes, breast cancer, atrial fibrillation, and UV exposure.
By providing real-time health data to patients—and in a format that’s be easily shareable with healthcare professionals—wearable devices can empower people to more closely manage chronic conditions and be more proactive about addressing previously undetected health issues (Fig. 1). According to Global Industry Analysts, Inc., at-home healthcare monitoring reduced hospital visits by 35% in 2017.
The idea behind many of these devices is to assess early indicators of certain conditions. To illustrate this point, let’s consider blood pressure—an important indicator of cardiovascular health and a vital sign that must be managed. The American Heart Association recommends that people with high blood pressure engage in home monitoring as a way to evaluate whether their treatments are effective. Plenty of home-monitoring tools are already available. Most of these are cuff-based and, as such, require dedicated time sitting down to capture the measurement. They also don’t provide a continuous reading.
For greater user convenience, wrist-based devices that monitor blood pressure continuously and in a non-invasive manner are starting to emerge. For example, Omron Healthcare’s HeartGuide, the first wearable blood-pressure monitor, has the approval of the U.S. Food and Drug Administration (FDA). HeartGuide is essentially a smart watch and uses the oscillometric cuff method, which is the standard for medical-grade personal blood pressure measurement.2 An accompanying app provides insight on readings and allows for sharing with the user’s doctor.
Accurate, Wrist-Based Blood-Pressure Monitoring
So, we can now measure blood pressure from a smart watch. How accurate are the readings? Accuracy has been a challenge in terms of blood-pressure monitoring from the wrist because the arteries in the wrist are narrower and not as deep under the skin as upper arm arteries. Also, the arm and wrist must be at heart level to capture a correct reading.3
One method of measuring blood pressure is via pulse transit time (PTT), which denotes the time that it takes a pulse pressure waveform to propagate through the length of the arterial tree. This pulse pressure waveform occurs when blood is ejected from the left ventricle, and it moves with a greater velocity than the forward movement of the blood itself. In addition to a measurement of blood pressure, PTT also provides an indicator of arterial stiffness.
PTT can be derived from calculations on electrocardiogram (ECG) and photoplethysmography (PPG) signals; it’s based on tightly defined characteristics of the shape of the pressure pulse waves in blood vessels.4 ECG provides a measurement as well as graphical representation, in terms of time, of the electrical signals associated with heart muscles. PPG provides an optical measurement of the volumetric change of blood in tissue during the cardiac cycle. Optical sensors that utilize PPG and ECG are used in wearables to measure heart rate. Measuring PTT involves calculating the time between the R-peak of the ECG and a reference point on the pressure pulse wave measured using PPG.5
Studies have shown, however, that measuring PTT alone to assess blood pressure may not be sufficient “because the regulation of blood pressure within the human body is a complex, multivariate physiological process,” notes a team of university and industry researchers in a paper published by the National Institutes of Health in its U.S. National Library of Medicine.6 In their study, the researchers found that integrating PTT, heart rate, and a previous blood-pressure estimate results in a more accurate current blood-pressure value. In addition to general blood-pressure monitoring, PTT has been examined for use in other applications, such as a means to detect sleep ailments via measurement of respiratory effort or as an indicator of the onset of low blood pressure during spinal anesthesia.
A Healthier World, One Data Point at a Time
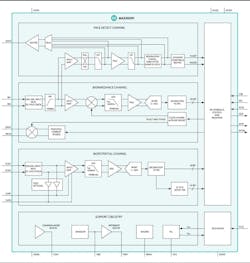
A variety of biosensor modules and biopotential analog front-ends (AFEs) are designed to be integrated into health wearables. Figure 2 shows an example. The biosensor modules integrate various components—LEDs, photodetectors, and low-noise electronics with ambient light rejection, for example—to enable mobile and wearable devices to measure ECG, pulse oximetry, and heart rate. The biopotential AFEs can be used to create solutions such as wearable health and fitness monitors that provide clinical-grade ECG monitoring.
Examinations were done that utilize a two-chip solution consisting of an ECG AFE and a PPG sensor to measure, via a wearable form factor, pulse arrival time (PAT), which is equal to the sum of PTT and the pre-ejection period. In this study, engineers configured the hardware and performed detailed register configuration settings to allow the two chips to acquire ECG and PPG data synchronously to derive PAT.
The ECG AFE’s interrupt registers were configured to generate a synchronization pulse after each ECG sample was placed in its data FIFO. The PPG sensor is configured to wake up with each ECG synchronization pulse and acquire a sample. After the PPG sensor completes a measurement and sets its FIFO interrupt, the micro-computer unit reads both the ECG and PPG FIFO data registers over an SPI interface. The process is repeated at the sample rate selected for the ECG AFE.
PAT is simpler to measure than PTT and, as a result, has been proposed as a PTT surrogate. Subsequent studies have shown that, due to accuracy issues, PAT isn’t an ideal replacement for PTT as a blood-pressure marker. However, it may potentially indicate wide trend blood-pressure changes in some cases.7 The interesting point here is, there’s a means using a two-chip solution to measure PAT via a wearable.
Now, imagine the potential solutions that could emerge by adding the capability to calculate PTT from the data gathered by the wearable form factors. Applying sensor fusion to bring together data from multiple sensors along with artificial intelligence to identify patterns and opportunities for action creates many more possibilities. The sophisticated data analysis would happen in the background. But for the wearer, he or she simply has a device that provides continuous monitoring, comfortably and conveniently.
According to the Tractica market intelligence firm, worldwide shipments of wearable healthcare devices are projected to hit nearly 98 million units annually by 2021.8 With increasing healthcare costs along with growing and aging populations, a good case can be made for tapping into the utility of wearable devices—and the rich data they collect—to transform the traditional healthcare model into one that’s more personalized, proactive, and preventive in nature.
Andrew Burt is Executive Business Manager, Industrial & Healthcare Business Unit, at Maxim Integrated.
References:
2. https://www.empr.com/home/news/fda-approved-wearable-blood-pressure-monitor-now-available/
4,5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5644691/
6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4512231/
7. https://www.frontiersin.org/articles/10.3389/fphys.2018.01848/full
About the Author
Andrew Burt
Executive Business Manager, Industrial & Healthcare Business Unit
Andrew Burt is an executive business manager at Maxim Integrated, where he focuses on business development for the company’s healthcare sensors, optical modules, and algorithms, and also helps define new sensors that will be part of future wellness and disease-management solutions. Andrew attended Oxford Brookes University, where he studied electrical and electronic engineering.