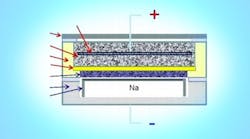
Sodium-beta batteries (Na-beta batteries or NBBs) are a traditional energy storage medium for power grid applications. NBBs use a solid beta-alumina (ß˝-Al2O3) electrolyte membrane that selectively allows sodium ion transport between a positive electrode (e.g., a metal halide) and a negative sodium electrode. Energy storage NBBs typically operate at temperatures near 350˚C, but they are used in utility applications due to their high charge-discharge efficiency, high energy densities, and energy storage capacities ranging from a few kilowatt-hours to multiple megawatt-hours. However, existing NBBs do not discharge stored electrochemical energy efficiently, because:
· They use thick tubular beta-alumina electrolytes that pass sodium between the anode and the cathode. Their cylindrical shape does not allow efficient discharge of stored electrochemical energy.
· A planar design would offer volumetric efficiencies, but requires robust seal development.
· Relatively high operating temperatures pose challenges for material selection and durability, making existing technology too expensive for a broader market penetration.
· Manufacturing processes for the high-temperature casing materials are expensive and difficult to automate.
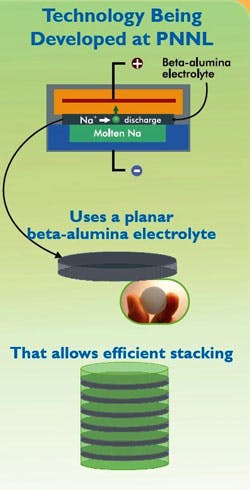
Now, a new material developed by the DoE’s Pacific Northwest National Laboratory (PNNL) should allow more utilities to store large amounts of renewable energy and make the power grid more reliable and resilient. It provides the following potential benefits:
· Uses a planar beta-alumina electrolyte to increase the active area and decrease the diffusion distance in the battery.
· Provides 30% higher energy density and 100% higher power density due to increased active area per volume and decreased diffusion distances
· Enables production of scalable, modular batteries at 50% of the cost of today’s tubular designs using inexpensive construction materials and manufacturing techniques
· Increases battery life because it operates at a lower temperature than traditional NBBs
· Enables manufacturing based on abundant, inexpensive materials
· Offers high charge-discharge efficiency and long cycle life
· Eliminates emissions during operation by using a completely sealed design
· Enables recycling of 99% of the battery materials; only sodium must be handled as a hazardous metal
By operating at significantly lower temperatures sodium-beta batteries will last longer, help streamline their manufacturing process and reduce the risk of accidental fire.
"Running at lower temperatures can make a big difference for sodium-beta batteries and should enable batteries to store more renewable energy and strengthen the power grid," said material scientist Xiaochuan Lu.
More than 300 megawatts of large, cargo container-sized sodium-beta batteries are running in the United States, Japan and Europe, according to Dupont Energy Consulting. They often store electricity generated by rows of solar panels and wind turbines.
Broader use of existing NBBs has been limited by their 350 °C operating temperature, more than three times the boiling point of water. These high operating temperatures require existing sodium-beta batteries to use more expensive materials, which shortens their operating lifespans. PNNL researchers set out to reduce the battery's operating temperature, knowing that it would make the battery more efficient and last longer.
Traditional sodium-beta batteries consist of two electrodes separated by a solid ceramic beta alumina membrane. There are two main types of sodium-beta batteries, based on the materials used for the positive electrode: those that use sulfur are called sodium-sulfur batteries, whereas those that use nickel chloride are known as ZEBRA batteries. The generate electricity when electrons flow between the battery's two electrodes.
The goal of lowering the battery's operating temperature poses several technical challenges. Key among them is getting the negative sodium electrode to fully coat, or "wet," the ceramic beta alumina membrane. Molten sodium resists covering beta alumina's surface when it's below 400 °C, causing sodium to curl up like a drop of oil in water and making the battery less efficient.
Lu and his PNNL colleagues, however, took an entirely different approach to the wettability problem: they modified the negative electrode. Instead of using pure sodium, they experimented with sodium alloys, or sodium blended with other metals. They determined that a liquid sodium-cesium alloy spreads out well on the beta alumina membrane.
PNNL's new electrode material enables the battery to operate at lower temperatures. Instead of the 350 °C at which traditional sodium-beta batteries operate, a test battery with the new electrode worked well at 150 °C, with a 420 mA-h per gram power capacity, matching the capacity of the traditional design.
Batteries with the new alloy electrode also retain more of their original energy storage capacity. After 100 charge and discharge cycles, a test battery with PNNL's electrode maintained about 97% of its initial storage capacity, whereas a battery with the traditional, sodium-only electrode maintained 70% after 60 cycles.
A battery with a lower operating temperature also uses less expensive materials such as polymers (which would melt at 350 °C) for its external casing instead of steel. Using less expensive and sensitive materials also helps streamline the battery's manufacturing process. This offsets some of the increased cost associated with using cesium, which is more expensive than sodium.
The PNNL research team is now building a larger electrode to test with a larger battery to bring the technology closer to the scale needed to store renewable energy.
This research was supported by DOE's Office of Electricity Delivery and Energy Reliability and internal PNNL funding. Project partners include the Pacific Northwest National Laboratory (www.pnl.gov), and EaglePicher Technologies, LLC (www.eaglepicher.com).