Researchers Look To Materials To Improve Micro-Grid Renewable Energy Storage
Researchers are hard at work looking to come up with a battery technology that meets the economic and consumer needs of a rapidly growing demand for renewable energy storage of the micro-grid. Energy would come from the sun, wind, batteries, and other sources. They’re examining promising results from batteries using liquid, molten metal elements, salt-water, iron flow, zinc, lithium-air, and other chemistries to satisfy the needs of the electrical grid, according to information from Grist (www.grist.org) and the U.S. Dept. of Energy (https://energy.gov/).
High cost is a big problem, according to Eric Rohlfing, deputy director of technology for ARPA-E, a division of the Department of Energy that identifies and funds leading-edge R&D. Since it was established by former President Obama in 2009, ARPA-E has funded $85 million toward developing new batteries that can meet that goal.
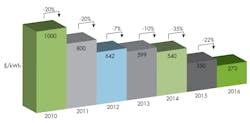
A 2012 study in Nature Magazine found that the average American would only be willing to pay about $13 more each month to ensure that the entire electrical grid could allow the U.S. electrical supply to run on renewable battery energy. That means utilities must be able to provide grid-level energy storage that would cost them less than $100 per kW-hour. According to Bloomberg New Energy Finance, battery prices continue to rapidly fall.
Presently, that rate is hundreds of $/kWh. According to many experts, it has to come down to under $100/kWh for batteries based on liquid, molten-metal, salt water, and other chemistries to be competitive in the market.
Battery prices continue to fall according to Bloomberg New Energy Finance, making it difficult for liquid, molten-metal, salt water, and other chemically based batteries to compete with an industry-standard baseline of $100/kWh. But progress toward that goal continues. (Source: bloomberg.com/news).
That hasn’t stopped researchers for trying to achieve that price goal. At laboratories at MIT, the Universities of Utah and Michigan, the U.S. Army Research Laboratory, and Harvard University, researchers are optimistic about their chances to hit that cost milestone.
Getting Market-Ready
Some success has been achieved getting renewable energy storage on the market. Aquion, a spinoff from Carnegie Mellon University, has its 35-megawatt-hour salt-water storage battery deployed around the world in 250 installations. Its goal is to produce the least expensive and most reliable battery one can make. “Our chemistry is very simple,” says Matt Maroon, Aquion’s vice president of product management. “It’s also very easy to assemble. Our main piece of manufacturing assembly comes out of the food industry” he adds.
Aquion claims that nothing in their battery is toxic, caustic, or flammable. All the materials in the Aquion batteries are abundant and easily obtained. One installation in Hawaii has been up and running for two years. Last year, the battery-plus-solar system powered several buildings for six months without ever falling back on a diesel generator.
Simple chemistry and simple assembly defines this saltwater Aquion solar-powered renewable energy battery. An installation in Hawaii has been up and running for two years, powering several buildings for six months without ever falling back on a diesel generator. (Source: Aquion).
Another spinoff company from MIT, Ambri, believes it literally has the next hot technology in energy storage: purified molten aluminum metal cells using a low-tech approach. Based in Marlborough, Mass., it was co-founded about a decade ago by materials chemistry professor Donald Sadoway and Ph.D. student David Bradwell. The improved materials run at a cool 900ºF, several hundred degrees lower than what has already been achieved by others. The Ambri process is doubled up and looped-back on itself. Some test cells have been running for nearly four years without showing any signs of wear and tear. The company solved some pesky heat-seal issues allowing battery packs to reach a self-sustaining operating temperature that’s hot enough to charge and discharge without an extra energy input. Ambri plans to begin production within two years.
This Ambri 432-cell 20-kWh working prototype runs itself using molten-metal chemistry to generate electricity. A commercial version of the same size could supply a day’s worth of electricity to 30 average Massachusetts homes. (Source: Ambri).
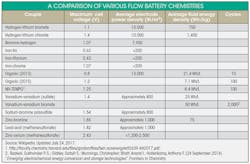
Using a predictive model of molecules and their properties, a Utah-Michigan Universities team of scientists developed a charge-storing molecule that is about 1,000 times more stable than current redox (relative oxidation) flow compounds in use today. The first compound had a half-life of about 8-12 hours (the time in which half the material would decompose). The scientists predicted that the compound would be stable on the order of months.
They used two tanks that hold the solutions containing molecules of charged atoms, called anolytes and catholytes, that store and release charge as the solution flows past the electrodes. To increase battery capacity, more material is added in the tanks and it flows through the same cell. By adding more cells, a faster rate of charge or discharge is achieved.
The scientists report that the most exciting anolyte is based on the organic molecule pryidium, which contains no metals and is intended to be dissolved in an organic solvent, further enhancing stability. Although other compounds exhibited longer half-lifes, the pyridium anolyte provides the best combination of stability and redox potential, which is directly related to how much energy it can store.
Interesting work at Harvard University makes use of predictive modeling of chemical structures as well as flow techniques. Professor Michael Aziz and graduate student Andrew Wong devised a small experimental storage system that relies on organic molecules from a class of compounds known as quinones. These can be found in rhubarb, which stores and transfers energy.
Electrochemical electrolyte ingredients are dissolved in water and stored in beakers. When these solutions flow near each other, they create an electric charge. They call their system an organic mega-flow battery, which can become a renewable energy source. These carbon-based solutions get recycled in the batteries, keeping carbon out of the atmosphere.
The compounds tried included aloe vera, vitamin K, nitrogen, and vitamin B2.Typically about a 1-W output was obtained. They do admit that it would take tanks twice the size of a Goodyear blimp to produce about a million W.
What’s Ahead?
As more of the aforementioned efforts in battery experimentations make headway, it may take quite a while before the battery that powers consumer electronics items becomes a reality. Let’s face it, the venerable battery technology most of us are familiar with will not improve much more, resulting in a smaller size, greater energy densities, greater reliability, and, of course, at an acceptable cost. It’s all about greater knowledge and application of chemistry. And flow battery technology, which has been around for four decades, may be one answer.
About the Author
Roger Allan
Roger Allan is an electronics journalism veteran, and served as Electronic Design's Executive Editor for 15 of those years. He has covered just about every technology beat from semiconductors, components, packaging and power devices, to communications, test and measurement, automotive electronics, robotics, medical electronics, military electronics, robotics, and industrial electronics. His specialties include MEMS and nanoelectronics technologies. He is a contributor to the McGraw Hill Annual Encyclopedia of Science and Technology. He is also a Life Senior Member of the IEEE and holds a BSEE from New York University's School of Engineering and Science. Roger has worked for major electronics magazines besides Electronic Design, including the IEEE Spectrum, Electronics, EDN, Electronic Products, and the British New Scientist. He also has working experience in the electronics industry as a design engineer in filters, power supplies and control systems.
After his retirement from Electronic Design Magazine, He has been extensively contributing articles for Penton’s Electronic Design, Power Electronics Technology, Energy Efficiency and Technology (EE&T) and Microwaves RF Magazine, covering all of the aforementioned electronics segments as well as energy efficiency, harvesting and related technologies. He has also contributed articles to other electronics technology magazines worldwide.
He is a “jack of all trades and a master in leading-edge technologies” like MEMS, nanolectronics, autonomous vehicles, artificial intelligence, military electronics, biometrics, implantable medical devices, and energy harvesting and related technologies.