Appropriate Package Technologies Enhance Medical Electronics
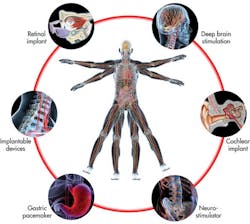
Medical electronics impact all of us, from sonograms of our babies to CT scans of broken wrists to a vast array of additional electronic devices designed to improve the quality of our lives. Some individuals even depend on electronics such as pacemakers and implanted defibrillators for vital regulatory functions.
An ever expanding array of implantable technology can supplement and supplant dysfunctional natural systems.
Whatever the application, we all rely upon the accuracy of the measurement results, diagnostics, and reliability of the equipment. The selection of the proper IC packaging technology strongly influences these goals, improving the form factor, performance, and reliability of the end medical equipment.
Many factors play into reliable medical electronics. For example, the environment is often fairly benign. The equipment used in a traditional doctor’s office won’t experience the environmental extremes of under-the-hood automotive or basestation electronics. Also, the electronics in implantable devices aren’t expected to withstand gravitational shocks like those experienced when users drop their phones onto concrete floors or down the stairs.
At the same time, medical electronics demand the newest technologies, including semiconductor packaging. CT scans deliver ever more resolution with faster image processing times, enabling 3D rendering, color coding of artifacts, and computer assisted diagnosis. Implantable devices become more compact and energy efficient with each design iteration. Pacemakers include ever more sophisticated DSP algorithms.
Even mass produced medical electronics such as glucometers are designed with the latest low-power microcontrollers. New packaging technologies such as wafer chip-scale packages shrink these implantable and consumer applications, opening new levels of portability, comfort, and applications such as artificial retinas and cochlear implants.
The Importance Of Reliability Testing
To achieve high levels of reliability, package technologies are carefully selected and characterized. Extended reliability testing can be used to ensure adequate lifetimes for medical devices. In an extended reliability test, a device population is tested well beyond the normal commercial requirements.
For some devices, the tests are continued until most of the devices have failed, providing valuable data from which acceleration rates are calculated. Other devices are subjected to more extreme conditions to provide a hammer test. Temperature cycling ranges can be extended from –40°C to 125°C to –55°C to 150°C or even –65°C to 150°C to provide a type of hammer test. Confidence limits can be increased through increased sample sizes.
Technologies for very high-reliability applications such as medical electronics should be selected to eliminate known failure mechanisms. For instance, tin (Sn) whiskers can grow on surfaces that are plated with Sn. These whiskers, which can be 400 mm to 500 mm long, can short across leads or break off and drop onto critical current paths.
Plating finishes such as nickel palladium gold (NiPdAu) can eliminate the Sn and the potential for Sn whiskers. Other risk mitigation actions include conformal coatings to seal surfaces against moisture and chemicals and package level underfills to reduce the risk of solder fatigue when temperature cycles, power cycles, or mechanical shocks are expected.
Packaging Considerations
Special package technologies are sometimes selected for medical electronics. For example, electronics in MRIs must operate in high magnetic field environments, so ferromagnetic materials such as Ni must be eliminated from these devices. For these packages, Sn finishes are often used.
Electronics in CT scanner detectors may require special shielding from any ionizing radiation. In computationally critical applications, low alpha particle emission materials are needed to eliminate the risk of logic upsets from the ionizing particles.
The electronic subsystem enclosure usually ensures biocompatibility in implantable devices as silicon by itself is neither biocompatible or biostable. Various body chemistries and tissues etch it, so it must be protected. Stainless steel is a common material for the cases of pacemakers and implantable defibrillators. Wire connections into these boxes are designed for bio-compatibility and hermeticity.
Bio-compatible materials for implantable electrode tips include tungsten, stainless steel, platinum/iridium, and pure iridium. These materials have multiple modes of interaction with various body and neural tissues and should be carefully selected for the application. Insulators for these electrodes are generally made from parylene or polyimide. All implantable devices must be able to survive sterilization in autoclaves before surgical insertion.
Many medical devices require careful documentation. FDA title 21 Quality System Regulation 820 details these requirements. ISO 13485 may also apply for ISO certified suppliers. These specifications require strict documentation of design, development testing, product testing, lot histories, field failures, and so on.
For example, one particular chip-scale package technology selected for medical electronics needed special controls such as enhanced verification and assessment of materials; auditing of incoming raw devices and printed wiring boards; special controls for stencil, placement, and reflow; increased audits and inspections; increased test accessibility for in-circuit testing; long-term yield trending; and training plans to qualify personnel.1
Medical devices are divided into classes, from 1 to 3. Devices in class 1 have the least restrictive qualification and documentation requirements due to their small potential for harm. Devices in class 3, such as pacemakers and other implants, have the most rigorous requirements. Additionally, guarantee of supply may be required so sufficient inventory exists to cover complete production and replacement lifetimes. These qualification and documentation requirements often make it difficult or cost prohibitive to change technologies during a product’s life, making the appropriate selection of technologies up-front even more essential for medical devices.
Building A Medical Infrastructure
An infrastructure of medical electronics suppliers has developed and continues to grow, offering compliance to standards, ensured supply, and the appropriate documentation to satisfy regulatory requirements. Specific events such as the Medical Design and Manufacturing Conference and the Design Med conference offer forums for professional development in all aspects of medical electronics design, including regulatory issues.
With life expectancy increases resulting in population aging on a global scale, growth in medical electronics is certain. New medical electronic technologies will be adopted at ever increasing rates to satisfy demands for improved quality of life in old age.
The proper selection of technologies, including semiconductor packaging solutions, and the right qualification and compatibility testing, coupled with ever increasing innovation in bio-electronics interfacing and bio-chemical analysis and feedback promise to make all of our futures brighter and more comfortable.
Reference
1. LeRoy Jarvis, “Implementing Chip-Scale Packaging in PCBA Medical Products,” Medical Electronics Design, April 01, 1999.
About the Author
Darvin Edwards
Darvin Edwards, TI Fellow, manages the SC Packaging modeling team at Texas Instruments. His team is responsible for electrical, thermal, and thermomechanical analysis of new products and package developments. He received his BS in physics from Arizona State University and holds 20 patents. He also has authored or co-authored over 45 papers, articles, and book chapters and has lectured on thermal challenges, modeling, reliability, electrostatic discharge (ESD), and 3D packaging