1. The Chevy Volt HEV contains a 192-cell Li-ion battery housed in a 5.5-foot-long T structure that weighs 183 kg. It’s manufactured by LG Chem and has a capacity of 18.4 kWh. (Courtesy of General Motors)
Although other technologies hold promise long-term, lithium-ion technology is the clear choice to power current-generation electric vehicles (EVs) (Fig. 1).
But unlike a traditional lead-acid battery, a Li-ion battery requires a considerable amount of care and feeding. Charging involves much more than simply hooking up an alternator. Even discharging past a certain point can lead to irreversible damage. This has led to the development of complex charging and discharging strategies at the level of the individual cell.
Why Lithium-Ion?
With an atomic number of 3, lithium is the lightest metal. It offers the greatest electrochemical potential and provides the largest specific energy per weight—both huge advantages for a battery. Unfortunately, metallic lithium is also unstable, flammable, and potentially explosive when exposed to air or water. Needless to say, battery research has concentrated on materials that use safer forms of the substance.
The positive electrode of a rechargeable Li-ion battery cell uses one of a variety of intercalated lithium compounds. These include lithium nickel manganese cobalt oxide ("NMC"), and lithium iron phosphate ("LFP"), each with slightly different characteristics. The negative electrode is most commonly made of graphite.
The liquid electrolyte consists of lithium salts in an organic solvent like ethylene carbonate or dimethyl carbonate. During operation, lithium ions move from the negative electrode to the positive electrode during discharging, and in the reverse direction during charging.
Lithium-ion has several advantages over earlier EV chemistries such as lead-acid and nickel-metal-hydride (NiMH). It’s lightweight, has no memory, and exhibits low self-discharge (around 1% per week). The nominal cell voltage is 3.6 V, vs. 1.5 V for NiMH and 2.0 V for lead-acid, so fewer cells have to be stacked in series to produce the high voltages needed for electric-vehicle motors.
We've come a long way in battery development since the early days of EVs. The battery in the Nissan Leaf, for example, contains 192 Li-ion cells with NMC and graphite electrodes. The cells are arranged in a 96 × 2 series/parallel array for 360-V nominal output and an energy density of 140 Wh/kg. In 1996, GM's EV1, the first mass-produced EV from a major manufacturer, used lead-acid batteries with a comparable output (312 V), but an energy density of only 31 Wh/kg.
Here Be Dragons
Li-ion batteries have many desirable features, but they’re much less tolerant than other chemistries to conditions such as overcharging, overdischarging, overtemperature, and excessive current.
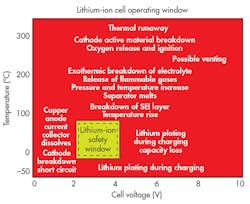
Excursions from the prescribed limits can have potentially catastrophic consequences, and not just in automotive. The Boeing 787 Dreamliner was grounded for three months in 2013 after two fires attributed to thermal runaway in its Li-ion batteries. Figure 2 shows the relatively small safe operating window.
2. Li-ion cells must be kept within a narrow range of temperatures and voltages to avoid failure. (Courtesy of US DOT)
Key Battery Parameters
In any vehicle that relies on its battery as part of the drivetrain, it's crucial that the battery-management system (BMS) continually monitors the condition of the battery, regardless of battery type. This is true across the board: micro-hybrids; conventionally powered vehicles that use lead-acid batteries for stop-start functions; hybrid EVs (HEVs), which use both conventional and electric motors; and battery EVs (BEVs), which are purely electrical.
Two parameters are commonly used to assess the state of a battery or cell:
State of charge (SoC): This is the equivalent of a fuel gauge in a conventional vehicle. It measures the available energy in the battery, from 0% (empty) to 100% (fully charged). The inverse metric is depth of discharge (DoD).
State of health (SoH): A figure of merit that measures the condition of a battery or cell compared to its ideal condition, unless the battery is out-of-specification as delivered. The SoH typically begins at 100%, then declines over time as the battery ages.
The BMS uses SoC and SoH to make decisions and regulate performance.
Charging and discharging of a battery occur via the terminals connected to each end of the series stack, not at the cell level. In lead-acid and NiMH chemistries, measurement and control of individual cells aren’t needed because they’re relatively insensitive to over and undercharging. Li-ion batteries, however, require a more sophisticated strategy.
Li-Ion Load Balancing
An EV battery employs hundreds of cells connected in series and in parallel to store sufficient energy for operation. The Nissan Leaf contains two modules of 96 Li-ion cells in series, and bus batteries may have over one hundred cells in series.
Within the battery, each individual cell, although very similar, isn’t identical. There are variations due to capacity, internal resistance, self-discharge rate, overvoltage threshold, thermal gradients in the battery, aging characteristics, and many other factors.
The consequence of these variations is that at any given time during the life of the battery, one cell (or small group of cells) in the battery will have a higher level of charge than the other cells. Similarly, another cell will have less charge than others.
For Li-ion, it's critical that no individual cell is overcharged or overdischarged to stay within the safe operating area. The charging of the whole battery stack must stop when the "high" cell reaches full charge, even though the other cells are at less than 100%. Likewise, discharging must come to an end when the "low" cell reaches empty, even though energy remains in other cells.
The entire battery’s performance will, therefore, be limited by the performance of only a couple of cells. In the charging cycle, the battery will never become fully charged; in the discharge cycle, energy goes to waste.
These cell imbalances will tend to increase over time. Eventually, both the charging and discharging performance will decrease below an acceptable level, requiring that the battery be replaced.
To combat this situation, battery-management designers have developed the technique of load balancing. It monitors individual cells and acts to lessen charge differences between them.
Measuring Cell Charge
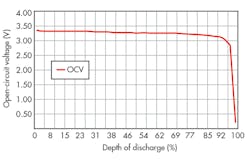
The charge on an individual cell can be determined by measuring its open-circuit voltage (OCV) and inferring the corresponding state of charge (or depth of discharge) from a graph similar to that shown in Figure 3. The results can be improved by applying correction factors derived from both current and temperature. Over the years, manufacturers have successively improved the performance so that a battery can maintain its output voltage over almost the full range of charge.
Ironically, this improvement has made it difficult for the control system to gain meaningful feedback. Since small differences in cell voltage can equate to large differences in charge, the voltage measurement must be accurate to within a couple of millivolts, which requires a high-precision analog-to-digital converter (ADC).
A 14-bit resolution ADC operating with a 5-V reference is a practical choice for a Li-ion cell with OCV of around 4.2 V. Typically, a single ADC measures the voltages of multiple cells, using a multiplexer to switch between channels. A successive-approximation-register (SAR) architecture is preferred because it has no latency between successive samples.
3. A Li-ion battery with a LFP electrode can maintain an almost constant OCV until 95% depth of discharge. (Courtesy of TI)
Once the charge on each cell is known, the load-balancing circuitry can act to equalize the charges. One of two approaches can be taken: passive balancing and active balancing.
Passive Load Balancing
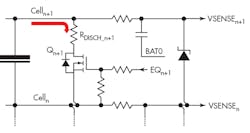
A passive load-balancing system draws energy from the most charged cell and dissipates it as heat, usually through a resistor. Figure 4 shows the circuitry for a single cell in a stack. The value of RDISCH is calculated based on the desired balancing current, the cell voltage, and the RDS(ON) of the FET switch.
The control algorithm, running on the BMS microcontroller, balances the charge in each cell by measuring its voltage VSENSE and discharging the cell if necessary until all cells are at the same voltage. The BMS also checks for battery-level fault conditions such as overtemperature, overvoltage, and undervoltage. Once all cells are balanced, the battery pack as a whole can be charged to fully charge each cell.
Active Load Balancing
Passive balancing is a one-way system which acts to reduce the energy in the most charged cell. Active balancing adds an extra level of sophistication. It still draws energy from the most-charged cell, but instead of simply dissipating it, the system transfers the charge to the least-charged cells through a number of bidirectional dc-dc converters. A microcontroller monitors the condition of all cells and determines which one should be charged or discharged.
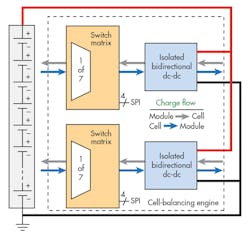
5. An active load-balancing system uses bidirectional dc-dc converters to source or sink current under control of the BMS microcontroller. (Courtesy of TI)
Figure 5 shows a block diagram of a typical active load-balancing engine.
The switch matrix allows for routing of charge into or out of a cell under control of the BMS microcontroller via SPI or other interface. The block connects the current from the dc-dc converter, which may be positive or negative, to the cell that needs to be charged or discharged. Multiple blocks may be used together to balance a larger stack of battery cells.
The isolated dc-dc converter exchanges energy between a single cell and the battery stack. Instead of using a resistor, the magnitude of the current is controlled by the load-balancing algorithm. The dc-dc converter block also checks for cell-level fault conditions.
Future Trends
The cost of battery packs for electric vehicles has decreased from $1000 per kilowatt-hour in 2007 to about $450 in 2014, with market-leading BEV manufacturers coming in at around at US$300 per kilowatt-hour. Forecasts show that the US$250 per kilowatt-hour point should be reached by around 2020, driven down further by economies of scale as home energy storage takes root.
Battery research is an active field, with university labs issuing announcements about the "next big thing" almost monthly. In the real world, though, Li-ion technology soldiers on, with slow but steady improvements in manufacturability and energy density—evolution rather than revolution.
The load-balancing electronics sector likewise shows characteristics of a mature market, with lower-cost products, integration of more features, and proliferation of options with new devices aimed at particular market segments.