Cobalt material is commonly used in the fabrication of rechargeable batteries for a wide range of consumer electronics devices, stationary energy storage units, electric vehicles, etc. Batteries’ cathode materials contain the rare metal cobalt, such as in the form of lithium cobalt oxide (LiCoO2), and according to a new market research report by Markets&Markets, the lithium ion battery market is expected to be valued at $69 billion by 2022, growing at a CAGR of 16.6% between 2016 and 2022.
A high demand of this mineral material is predicted; however, there are concerns about insufficient supply and rising costs of cobalt. Companies using cobalt have few supplier options because 60% of the world’s cobalt is mined in one place: The Democratic Republic of Congo (DRC). It is worth noting that 97% of the world’s supply of cobalt comes as a byproduct of nickel or copper. Also, cobalt mining conditions reports by Amnesty International are putting end users like Apple under pressure to take actions by suspending purchases from some suppliers in the DRC because of poor working conditions.
Based on concerns about insufficient supply and the rising cost of cobalt, Fujitsu Laboratories has been developing materials that use iron, which is abundant on Earth, as a constituent element in place of cobalt. They had successfully developed a cathode material for lithium iron phosphate rechargeable batteries capable of achieving high voltages that could only be achieved by cobalt-based materials in the past. More specifically, Fujitsu Laboratories has successfully synthesized lithium iron pyrophosphate (Li5.33Fe5.33(P2O7)4); this phosphate-based material has a voltage of 3.8 V, comparable to that of existing cobalt-based materials.
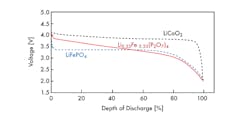
Curve of “Voltage vs. Depth of discharge” of a prototype coin battery using (Li5.33Fe5.33(P2O7)4) compare to previously developed materials. (Courtesy of Fujitsu)
Previously, there was a problem with lithium-ion batteries using iron-based materials as they could not reach the energy density of those using cobalt-based materials. Accordingly, iron-based materials with voltage of 2.8 V to 3.5 V could not compete with cobalt-based materials whose voltage ranged from 3.75 V to 4.1 V. It is known that the voltage of cathode materials can change depending on the arrangement of atoms in the crystal structure, which created issues in the development of new iron-based materials with high voltage.
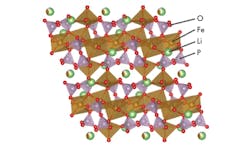
Fujitsu created advanced design of new crystal structure that can maintain a high voltage state for longer periods. The voltage of cathode materials is significantly influenced by the coordination of elements such as iron and oxygen in the crystal. By analyzing the interrelationship between the crystal structure of a material and its electrochemical characteristics, it was discovered that a distorted arrangement of oxygen atoms around iron atoms is one of the critical factors for the high voltage.
Here is a crystal structure of the new material. (Courtesy of Fujitsu)
Fujitsu Laboratories created a coin-shaped prototype battery and, based on the results of its electrochemical properties evaluation, it was confirmed that it could achieve a voltage of 3.8 V, comparable to that of existing cobalt-based materials.
This prototype coin-shaped battery has a charge capacity of 105 mAh/g. (Courtesy of Fujitsu)
The newly developed cathode material has not reached a voltage equal to existing cobalt-based materials in terms of energy density but Fujitsu Laboratories will work to design a crystal structure that can maintain a voltage on par with cobalt-based materials for longer periods. The electrode can also be used as a low-cost cathode material in safe, solid-state rechargeable batteries.
If a cathode material is developed with the same energy density as cobalt-based materials, this will enable the stable production of cathode materials by replacing the rare metal cobalt with abundant iron. Moreover, this is expected to contribute to the stable production of lithium-ion batteries. In the future, we will see more companies like Fujitsu Laboratories that will contribute to a more sustainable and comfortable society by developing next-generation high-energy-density rechargeable batteries that are safer, cheaper and environmentally friendly.