Making Electronic Products More Recyclable
This article is part of the System Design Series: RoHS and Critical Materials
Members can download this article in PDF format.
What you’ll learn:
- What SVHC metals and non-metals currently have no commercially viable alternatives?
- Issues with meeting EPEAT certifications and maintaining product design performance.
- Basic principles for electronics recycling.
The European Union (EU) led the way to enforcing collection and recyclability goals back in 2003 with its WEEE Directive (Waste Electrical and Electronic Equipment). Shortly thereafter, the EU’s Eco-Design Directive of 2008 published guidelines for product designs that consider the entire product lifecycle. These measures were further solidified with the 2018 amendment to the EU Waste Framework Directive (WFD), which began to strongly promote the development of a Circular Economy.
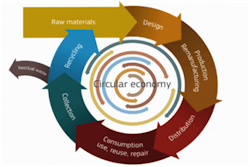
Some of the key goals of the Circular Economy include reducing the hazardous substances that may end up as waste by supporting the substitution of substances of concern, and to make information on product content available to waste treatment operators and recyclers. The figure shows a product lifecycle from raw materials through design and production, product use, reuse if possible, followed by collection and recycling back into the same circle. Only a small residual waste output is shown on the left.
Source: https://ec.europa.eu/easme/en/news/r2-supporting-transition-circular-economy. Reprinted without modification under http://creativecommons.org/licenses/by/4.0/. © European Union, 1995-2021
Voluntary environmental certification programs for certain products are also in place- and in fact are not voluntary at all if the customer requires compliance with them for procurement. Among them is the EPEAT program for high-tech electronics managed by the Global Electronics Council. The following types of equipment are currently covered, listed here with their associated industry standards.
- Computers and displays: IEEE 1680.1
- Imaging equipment: IEEE 1680.2
- TVs: IEEE 1680.3
- Mobile phones: UL 110
- Photovoltaic modules and inverters: NSF/ANSI 457
- Servers: NSF/ANSI 426-2018
These types of certifications use a series of evaluations that allow the manufacturer to earn points to meet minimum criteria. The questions include attention to materials selection during product design to avoid toxic substances and materials, and consideration for other types of negative environmental impact. Ability of the product to be reused and then recycled at end-of-life is another category earning points toward certification.
Ten groups of substances have already been regulated to less than 0.1% (0.01% for cadmium and its compounds) by the EU’s RoHS Directive (Restriction of Hazardous Substances). Since many manufacturers operate globally, these restrictions have been widely adopted by the major OEMs.
However, for those companies not doing business in the EU marketplace, and in fact all who require the use of RoHS Exemptions for product performance and reliability, some hazardous substances may still be present. In reality, most of the remaining substances cannot simply be designed out or replaced with choices available to today’s product designer.
As of January 2021, products entering the EU market must now publicly declare Substances of Very High Concern (SVHC) in a new database called SCIP (Substances Contained In Products) (see “New EU Reporting Requirements for Substances of Very High Concern”).
Most electronics engineers will probably avoid looking up the full SVHC list of some 211 such substances under the EU REACH Directive (European Chemicals Agency Registration, Evaluation and Authorization of CHemicals). But chances are that someone in your company will, and what follows is offered as practical information that you should know now.
A Closer Look at SVHC Candidates
Two metals in particular—lead and cadmium—are both banned by RoHS and listed as SVHC under REACH. However, they’re still needed in small amounts for specific types of device performance and reliability that may be allowed under specific RoHS Exemptions. Here’s a short list of some of the more common usages of these metals in component parts for which there are presently no commercially viable substitutes:
- Lead in machinable steel alloys up to 0.35% per homogenous material.
- Lead in machinable aluminum alloys up to 0.4% per homogenous material.
- Lead in copper alloys like brass up to 4.0% per homogenous material.
- Lead in glass, in which case lead is present in an oxidized form combined with other substances bound within the glass.
- Lead in high-temperature solder, used for reliability in components like power semiconductors, and in applications where a two-temperature soldering process is required.
- Lead in ceramic components like lead zirconium-titanate (PZT) piezoelectric sensors and transducers.
- Cadmium in alloys like silver cadmium oxide for high-current switch and relay contacts.
Non-metals that are SVHC may also be found in certain applications:
- Remaining non-RoHS restricted phthalate plasticizers in soft materials and polymers like polyvinylchloride (PVC). These may typically include wire and cable insulation and gaskets.
- Various plastics may require the use of flame retardants for fire safety that are SVHC but not the RoHS-restricted type. Dechlorane Plus is one example.
- Siloxanes (called D4, D5 and D6) that are low level residues from the manufacturing of polysiloxane polymers may be found in gaskets and sealants.
- Perfluoro compounds, like perfluorobutane sulfonic acid (PFBS). Small amounts may remain from the manufacturing of non-stick, self-lubricating plastics or other end products that contain fluoropolymers.
Design for Environment Gaps
Environmental product certifications like EPEAT require that the lifecycle aspects of the product be considered, including end-of-life and materials recovery. At the product level, the designer is faced with limited choices when it comes to ensuring that, first, the product will perform as intended in the field and meet its stated reliability goals. Even basic consumer electronics goods must withstand some shock and vibration, variations in temperature, and so on.
This raises a fundamental engineering challenge: How to ensure the integrity of the product during use yet allow it to be easily disassembled and fractionated into separate materials by a recycler.
At the component level, such as passives, discrete semiconductors and ICs, beyond “lead-free” terminations, and some devices inherently containing no SVHC at all, no truly great alternatives are possible. Consider the nature of the integrated circuit itself: Multiple materials are deposited in thin films onto a substrate, which is further packaged in a housing of thermoset plastic, or at minimum provided with solderable bumps. There’s no practical design choice here for a “recyclable” chip, at least not now.
Similarly, the designer has few if any choices in printed wiring board (PWB) selection. Some choices are available in the flame retardant used that may avoid bromine or other halogens. Research has been done on expensive processes to separate the glass filler and metals from the PWB itself to chemically recycle the laminate, but these possible approaches aren’t yet widely adopted, and even so are intended to work on today’s materials. Flexible circuits may be mounted directly to a metal housing, which has disassembly problems of its own. And a traditional hybrid has the same problems with diverse materials typically adhered to an alumina substrate.
Even if a main PWB assembly itself can be made removable, manual disassembly isn’t likely to occur in a practical high-volume recycling operation, especially for smaller products. Today’s products with glass displays also present the problem of recycling the glass itself, not to mention thin-film semiconductors like LEDs that are integral to displays.
A similar dilemma exists with touchscreens using visually transparent indium-tin-oxide capacitive touch sensors. Only larger products with separate metal enclosures are somewhat better from the perspective of possible separation into useful recycling streams before further processing.
Today Isn’t So Bad After All
The fundamental issue with recyclable electronics is really the need to have both conductors (or semiconductors) and insulators present at the same time. One without the other can’t enable even basic electricity to work and work safely. And for practical products, they must be in close and robust contact either through internal manufacturing processes, molding, extrusion, or assembly with solder or adhesives.
Conductors tend to be based on metals, while insulators are generally carbon-based (defined as “organic” by chemists). What if the conductive materials could also be based on carbon? In fact, such materials are being researched and include candidates like carbon nanotubes; polymers like polyacetylene, polypyrrole, polyaniline, polyphenylene sulfide, PEDOT (polyethylenedioxythiophene); and TCNQ (tetracyanoquinodimethane).
Besides the fact that those are generally not ready to be picked up and used by the designer, they would bring another even worse outcome—now they could not readily be separated at all for recycling, unlike metallic and organic materials. Plastic materials with conductive properties would have limited reuse potential.
Basic Principles and Recommendations
The keys to recycling today are based on a few basic principles that actually work:
- Density: The ability of hardware to be shredded and shrunk down allows for sorting into useful fractions that can be further treated for material recovery. Very-low-density materials like labels and fillers can then be floated to the top or picked off of the rest of the stream.
- Temperature: Metals melt only at high temperature above which most organics can’t survive without burning. The different melting properties of different metals further enables their separation back to useful pure metals or alloys by dedicated metal smelters. An all-carbon-based assembly could not take advantage of this.
- Special properties: Ferrous alloys can be sorted by magnets. Aluminum and other fractions are sortable with eddy-current technology. In some cases, optical sorting can be used to further sort other materials like plastics into useful fractions.
In short, keeping metals around as conductors and carbon, glass, or ceramics as insulators isn’t really a bad idea. And the going rate for metal scrap can give positive financial returns.
With all of this in mind, it seems we’re left with only a few recommendations for the electronic product designer today:
- Continue to search for the components and materials with the lowest substance of concern content and lowest environmental footprint whenever possible.
- Ask for such components and materials from your suppliers to create demand.
- Small substitutions in additives to raw materials may be possible; for example, some of the SVHC used to enhance polymer products that aren’t critical to performance may in fact have alternatives available.
- Think out of the box on how your product could use totally new approaches; for example, instead of subtractive processes like machining, consider additive processes such as 3D printing.
- Since the author has no other grand magic bullets to offer at this time, every small step forward is valuable. But don’t take this short article as the last word. Take up the challenge and help blaze a bigger trail to the circular economy.
About the Author
Roger L. Franz
Environmental Sustainability Advocate and Contributing Author
Roger Franz has served as Principal, Engineering IT at TE Connectivity, Research Engineer at UL Environment, and Group Leader and Design for Environment Manager at Motorola. In addition to product environmental compliance and standards, his passions include new product development, quality, and technology research. His papers have appeared in several industry journals. He has had over a dozen article published in Electronic Design. He holds a BA and MS in chemistry from Grinnell College and Northwestern University, respectively.