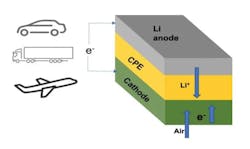
Lithium-Air Could Replace Li-ion Batteries to Significantly Boost EV Range
Arguably the toughest hurdle facing EV designers is creating a battery pack that could power a vehicle for as much as 1,000 miles on a single charge. With that goal in mind, researchers at the Illinois Institute of Technology (IIT) and U.S. Department of Energy’s (DoE) Argonne National Laboratory developed a lithium-air battery that could make this dream a reality. The team’s new battery design could also one day power airplanes and long-haul trucks.
The lithium-air battery uses a solid electrolyte instead of the typical liquid variety, potentially boosting the battery’s energy density by as much as 4X above Li-ion batteries, which translates into a longer driving range. The battery also reflects a commitment to push boundaries as far as they can go in any given direction. In addition, the new design isn’t subject to the usual safety issues with the liquid electrolytes used in Li-ion and other batteries, which can overheat and catch fire.
Lithium-oxide formation involves a four-electron reaction that’s more difficult to achieve than the one- and two-electron reaction processes that result in lithium superoxide (LiO2) and lithium peroxide (Li2O2) at the cathode. According to the team, its lithium-air design is the first lithium-air battery to achieve a four-electron reaction at room temperature. It also operates with oxygen supplied by air from the surrounding environment. The capability to run with air avoids the need for oxygen tanks to operate, a problem with earlier designs.
Solid Electrolyte
The team’s new solid electrolyte truly distances it from other battery chemistries. It’s composed of a ceramic polymer material made from relatively inexpensive elements in nanoparticle form. This new solid enables chemical reactions that produce lithium oxide (Li2O) on discharge.
The lithium peroxide or superoxide is then broken back down into its lithium and oxygen components during the charge. This chemical sequence stores and releases energy on demand.
“The chemical reaction for lithium superoxide or peroxide only involves one or two electrons stored per oxygen molecule, whereas that for lithium oxide involves four electrons,” said Argonne chemist Rachid Amine. More electrons that are stored means higher energy density.
Electrocatalysis (the use of catalysts to modify the rates of electrochemical reactions) of the four-electron oxygen reduction reaction (ORR) promises to play a key role in the development of sustainable and clean energy technologies, particularly when related to energy storage and conversion devices. The term “oxygen reduction reaction” refers to the reduction reaction whereby O2 is reduced to water or hydrogen peroxide.
Although the team hasn’t nailed down exact numbers, “with further development, we expect our new design for the lithium-air battery to also reach a record energy density of 1,200 watt-hours per kilogram,” said Larry Curtiss, an Argonne Distinguished Fellow. “That is nearly four times better than lithium-ion batteries.”
Past lithium-air test cells also suffered from very short lifecycles. As you might expect, the team established that this shortcoming isn’t the case for their new battery design by building and operating a test cell for 1,000 cycles, demonstrating its stability over repeated charging and discharging.
The research, published in a recent issue of Science, was funded by the DoE Vehicle Technologies Office and the Office of Basic Energy Sciences through the Joint Center for Energy Storage Research.