This articles is part of the Power Management Series in the Power Management section of our Series Library.
Download this article as a .PDF eBook.
Fuel cells convert the chemical energy from a fuel into electricity through a chemical reaction of positively charged hydrogen ions with oxygen or another oxidizing agent. They differ from batteries because they require a continuous source of fuel and oxygen or air to sustain the chemical reaction. Fuel cells produce electricity continuously as long as these inputs are supplied. In a battery, the chemicals present in the battery react with each other to generate a voltage. They are used for primary and backup power for commercial, industrial, and residential buildings and in remote or inaccessible areas. They can also be used to power vehicles, including forklifts, automobiles, buses, boats, motorcycles, and submarines.
All fuel cells consist of an anode, cathode, and electrolyte that allows positively charged hydrogen ions (or protons) to move between the two sides of the fuel cell. The anode and cathode contain catalysts that cause the fuel to undergo oxidation reactions that generate positively charged hydrogen ions and electrons. The hydrogen ions are drawn through the electrolyte after the reaction. At the same time, electrons are drawn from the anode to the cathode through an external circuit, producing direct current electricity. At the cathode, hydrogen ions, electrons, and oxygen react to form water.
Individual fuel cells produce relatively small electrical potentials, about 0.7 V, so cells are “stacked,” or placed in series, to create sufficient voltage to meet an application’s requirements. Besides electricity, fuel cells produce water, heat, and, depending on the fuel source, very small amounts of nitrogen dioxide and other emissions. The energy efficiency of a fuel cell is generally between 40% to 60%, or up to 85% efficient in cogeneration if waste heat is captured for use. The most widely used types are:
• Proton Exchange Membrane Fuel Cells (PEMFC)
• Solid Oxide Fuel Cells (SOFC)
Fuel-Cell Applications
Automobiles—As of 2015, two fuel-cell vehicles have been introduced for commercial lease and sale in limited quantities: the Toyota Mirai and the Hyundai ix35 FCEV. Additional demonstration models include the Honda FCX Clarity and Mercedes-Benz F-Cell. General Motors and its partners estimated that per mile traveled, a fuel-cell electric vehicle running on compressed gaseous hydrogen produced from natural gas could use about 40% less energy and emit 45% less greenhouse gases than an internal combustion vehicle. A lead engineer from the Department of Energy whose team is testing fuel-cell cars said in 2011 that the potential appeal is that “these are full-function vehicles with no limitations on range or refueling rate so they are a direct replacement for any vehicle.”
Forklifts—Fuel-cell forklifts lift and transport materials. In 2013, there were over 4,000 fuel-cell forklifts used in material handling in the United States. Most companies in Europe and the U.S. do not use petroleum-powered forklifts, as these vehicles work indoors where emissions must be controlled and instead use electric forklifts. Fuel-cell-powered forklifts can provide benefits over battery-powered forklifts as they can work for a full eight-hour shift on a single tank of hydrogen and can be refueled in three minutes. Fuel-cell-powered forklifts can be used in refrigerated warehouses, because lower temperatures do not degrade their performance.
Motorcycles and bicycles—In 2005, a British manufacturer of hydrogen-powered fuel cells, Intelligent Energy (IE), produced the first working hydrogen-run motorcycle called the ENV (Emission Neutral Vehicle). The motorcycle holds enough fuel to run for four hours, and to travel 160 km (100 mi) in an urban area, at a top speed of 80 km/h (50 mph).
Airplanes—Boeing researchers and industry partners throughout Europe conducted experimental flight tests in February 2008 of a manned airplane powered only by a fuel cell and lightweight batteries. The fuel-cell demonstrator airplane, as it was called, used a (PEM) fuel-cell/lithium-ion battery hybrid system to power an electric motor, which was coupled to a conventional propeller. In 2003, the world’s first propeller-driven airplane to be powered entirely by a fuel cell was flown. The fuel cell was a stack design that allowed it to be integrated with the plane’s aerodynamic surfaces.
UAVs—A Horizon fuel-cell UAV set the record distance flown for a small UAV in 2007. The military is interested in this application because of its low noise, low thermal signature, and ability to attain high altitude. In 2009, the Naval Research Laboratory’s (NRL’s) Ion Tiger utilized a hydrogen-powered fuel cell and flew for 23 hours and 17 minutes. Fuel cells are also in use to provide auxiliary power in aircraft, replacing fossil-fuel generators that were previously used to start the engines and power on-board electrical needs.
Boats—The HYDRA fuel-cell boat used an AFC system with 6.5 kW net output. Iceland committed to converting its vast fishing fleet to use fuel cells to provide auxiliary power and, eventually, to provide primary power in its boats. Amsterdam recently introduced its first fuel-cell-powered boat that ferries people around the city’s canals.
Submarines—German and Italian submarines use fuel cells to remain submerged for weeks without the need to surface. The U212A is a non-nuclear submarine developed by German naval shipyard Howaldtswerke Deutsche Werft. The system consists of nine PEM fuel cells, providing between 30 kW and 50 kW each. The ship is silent, giving it an advantage in the detection of other submarines.
Portable Power Systems—Fuel cells can be used in the leisure sector (i.e., RVs, cabins, marine), the industrial sector (i.e., power for remote locations including gas/oil wellsites, communication towers, security, weather stations), and in the military sector. SFC Energy is a German manufacturer of direct methanol fuel cells for a variety of portable power systems. Ensol Systems Inc. is an integrator of portable power systems, using the SFC Energy DMFC.
The most important design features in a fuel cell are:
• The electrolyte substance. The electrolyte substance usually defines the type of fuel cell.
• The fuel that is used. The most common fuel is hydrogen.
• The anode catalyst breaks down the fuel into electrons and ions. The anode catalyst is usually made up of very fine platinum powder.
• The cathode catalyst turns the ions into waste chemicals like water or carbon dioxide. The cathode catalyst is often made up of nickel, but it can also be a nanomaterial-based catalyst.
A typical fuel cell produces a voltage from 0.6 V to 0.7 V at full-rated load. Voltage decreases as current increases, due to several factors:
• Activation loss.
• Ohmic loss (voltage drop due to resistance of the cell components and interconnections).
• Mass transport loss (depletion of reactants at catalyst sites under high loads, causing rapid loss of voltage).
To deliver the desired amount of energy, the fuel cells can be combined in series to yield higher voltage, and in parallel to allow a higher current to be supplied. Such a design is called a “fuel-cell stack.” The cell surface area can also be increased, to allow higher current from each cell. Within the stack, reactant gases must be distributed uniformly over each of the cells to maximize the power output.
Proton Exchange Membrane Fuel Cells (PEMFCs)
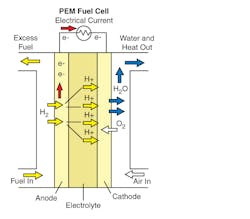
In the archetypical hydrogen-oxide proton-exchange membrane fuel-cell design, a proton-conducting polymer membrane contains the electrolyte solution that separates the anode and cathode sides (Fig. 22-1).
22-1. Proton exchange membrane fuel cell
On the anode side, hydrogen diffuses to the anode catalyst where it later dissociates into protons and electrons. These protons often react with oxidants, causing them to become what are commonly referred to as multi-facilitated proton membranes. The protons are conducted through the membrane to the cathode, but the electrons are forced to travel in an external circuit (supplying power) because the membrane is electrically insulating. On the cathode catalyst, oxygen molecules react with the electrons (which have traveled through the external circuit) and protons to form water.
The materials used for different parts of the fuel cells differ by type. The bipolar plates may be made of different types of materials, such as, metal, coated metal, graphite, flexible graphite, C–C composite, carbon–polymer composites, etc. The membrane electrode assembly (MEA) is referred as the heart of the PEMFC and is usually made of a proton exchange membrane sandwiched between two catalyst-coated carbon papers. Platinum and/or similar type of noble metals are usually used as the catalyst for PEMFC. The electrolyte could be a polymer membrane.
Phosphoric Acid Fuel Cell (PAFC)
In these cells, phosphoric acid is used as a non-conductive electrolyte to pass positive hydrogen ions from the anode to the cathode. These cells commonly work in temperatures of 150°C o 200°C. This high temperature will cause heat and energy loss if the heat is not removed and used properly. This heat can be used to produce steam for air-conditioning systems or any other thermal energy-consuming system. Using this heat in cogeneration can enhance the efficiency of phosphoric acid fuel cells from 40% to 50% up to about 80%. Phosphoric acid, the electrolyte used in PAFCs, is a non-conductive liquid acid that forces electrons to travel from anode to cathode through an external electrical circuit. Since the hydrogen ion production rate on the anode is small, platinum is used as a catalyst to increase this ionization rate. A key disadvantage of these cells is the use of an acidic electrolyte. This increases the corrosion or oxidation of components exposed to phosphoric acid.
Solid Oxide Fuel Cells
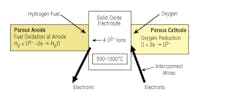
Solid oxide fuel cells (SOFCs) use a solid material, most commonly a ceramic material called yttria-stabilized zirconia (YSZ), as the electrolyte (Fig. 22-2). Because SOFCs are made entirely of solid materials, they are not limited to the flat-plane configuration of other types of fuel cells and are often designed as rolled tubes. They require high operating temperatures (800°C-1,000°C) and can be run on a variety of fuels including natural gas.
22-2. Solid oxide fuel cell.
SOFCs are unique in that negatively charged oxygen ions travel from the cathode (positive side of the fuel cell) to the anode (negative side of the fuel cell) instead of positively charged hydrogen ions traveling from the anode to the cathode, as is the case in all other types of fuel cells. Oxygen gas is fed through the cathode, where it absorbs electrons to create oxygen ions. The oxygen ions then travel through the electrolyte to react with hydrogen gas at the anode. The reaction at the anode produces electricity and water as byproducts. Carbon dioxide may also be a byproduct depending on the fuel, but the carbon emissions from an SOFC system are less than those from a fossil-fuel combustion plant.
SOFC systems can run on fuels other than pure hydrogen gas. However, since hydrogen is necessary for the reactions listed above, the fuel selected must contain hydrogen atoms. For the fuel cell to operate, the fuel must be converted into pure hydrogen gas. SOFCs are capable of internally reforming light hydrocarbons such as methane (natural gas), propane, and butane. These fuel cells are at an early stage of development.
Challenges exist in SOFC systems due to their high operating temperatures. One such challenge is the potential for carbon dust to build up on the anode, which slows down the internal reforming process. Research to address this “carbon coking” issue at the University of Pennsylvania has shown that the use of copper-based cermet (heat-resistant materials made of ceramic and metal) can reduce coking and the loss of performance. Another disadvantage of SOFC systems is slow startup time, making SOFCs less useful for mobile applications. Despite these disadvantages, a high operating temperature provides an advantage by removing the need for a precious metal catalyst like platinum, thereby reducing cost. Additionally, waste heat from SOFC systems may be captured and reused, increasing the theoretical overall efficiency to as high as 80% to 85%.
The high operating temperature is largely due to the physical properties of the YSZ electrolyte. As temperature decreases, so does the ionic conductivity of YSZ. Therefore, to obtain optimum performance of the fuel cell, a high operating temperature is required. According to its website, Ceres Power, a UK-based SOFC fuel-cell manufacturer, has developed a method of reducing the operating temperature of their SOFC system to 500°C to 600°C. They replaced the commonly used YSZ electrolyte with a CGO (cerium gadolinium oxide) electrolyte. The lower operating temperature allows Ceres Power to use stainless steel instead of ceramic as the cell substrate, which reduces cost and startup time of the system.
Theoretical Maximum Efficiency
The energy efficiency of a system or device that converts energy is measured by the ratio of the amount of useful energy put out by the system (output energy) to the total amount of energy that is put in (input energy) or by useful output energy as a percentage of the total input energy. In the case of fuel cells, useful output energy is measured in electrical energy produced by the system. Input energy is the energy stored in the fuel. According to the U.S. Department of Energy, fuel cells are generally between 40% to 60% energy-efficient. This is higher than some other systems for energy generation. For example, the typical internal combustion engine of a car is about 25% energy-efficient. In combined heat and power (CHP) systems, the heat produced by the fuel cell is captured and put to use, increasing the efficiency of the system to up to 85%–90%.
The theoretical maximum efficiency of any type of power generation system is never reached in practice, and it does not consider other steps in power generation, such as production, transportation, and storage of fuel and conversion of the electricity into mechanical power. However, this calculation allows the comparison of different types of power generation. The maximum theoretical energy efficiency of a fuel cell is 83%, operating at low power density and using pure hydrogen and oxygen as reactants (assuming no heat recapture). According to the World Energy Council, this compares with a maximum theoretical efficiency of 58% for internal combustion engines. While these efficiencies are not approached in most real-world applications, high-temperature fuel cells (solid oxide fuel cells or molten carbonate fuel cells) can theoretically be combined with gas turbines to allow stationary fuel cells to come closer to the theoretical limit. A gas turbine would capture heat from the fuel cell and turn it into mechanical energy to increase the fuel cell’s operational efficiency. This solution has been predicted to increase total efficiency to as much as 80%.
Solid-oxide fuel cells produce exothermic heat from the recombination of the oxygen and hydrogen. The ceramic can run as hot as 800°C. This heat can be captured and used to heat water in a micro combined heat and power (m-CHP) application. When the heat is captured, total efficiency can reach 80% to 90% at the unit, but does not consider production and distribution losses. CHP units are being developed today for the European home market.
Power
Stationary fuel cells are used for commercial, industrial, and residential primary and backup power generation. Fuel cells are very useful as power sources in remote locations, such as spacecraft, remote weather stations, large parks, communications centers, rural locations including research stations, and in certain military applications. A fuel-cell system running on hydrogen can be compact and lightweight, and has no major moving parts. Because fuel cells have no moving parts and do not involve combustion, in ideal conditions they can achieve up to 99.9999% reliability. This equates to less than one minute of downtime in a six-year period.
Since fuel-cell electrolyzer systems do not store fuel in themselves, but rather rely on external storage units, they can be successfully applied in large-scale energy storage, rural areas being one example. There are many different types of stationary fuel cells so efficiencies vary, but most are between 40% and 60% energy-efficient. However, when the fuel cell’s waste heat is used to heat a building in a cogeneration system, this efficiency can increase to 85%. This is significantly more efficient than traditional coal power plants, which are only about one third energy-efficient. Assuming production at scale, fuel cells could save 20% to 40% on energy costs when used in cogeneration systems. Fuel cells are also much cleaner than traditional power generation; a fuel-cell power plant using natural gas as a hydrogen source would create less than one ounce of pollution (other than CO2) for every 1,000 kW·h produced, compared to 25 pounds of pollutants generated by conventional combustion systems. Fuel cells also produce 97% less nitrogen oxide emissions than conventional coal-fired power plants.
Steel Cell
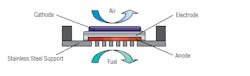
Ceres Power Holdings Plc’s steel-cell technology uses the existing infrastructure of natural gas mains and is manufactured using commodity materials such as steel and standard processes already used in the photovoltaic industry, meaning that it can be mass produced at an affordable price for domestic, business, and other applications in multiple markets.
The patented Ceres steel cell (Fig. 22-3) is a combination of unique technology, durable low-cost materials, and existing manufacturing processes. The steel cell is unique because it operates at temperatures of 500° C to 600°C, allowing use of low-cost steel and abundant ceramics with cost-effective mass manufacturing, at the same time as delivering high performance.
22-3. Ceres Steel Cell combines unique technology, durable low-cost materials, and existing manufacturing processes.
The steel cell is made by screen printing layers of ceramic ink onto a drilled sheet of steel. Achieving these high-quality ceramic layers at low temperature on steel is protected through extensive registered intellectual property and know-how. Exclusive to Ceres is the use of Ceria in the anode and electrolyte. Ceria is as abundant as copper and is used industrially for dyeing glass, self-cleaning ovens, and catalytic converters in cars. Steel needs no introduction as the backbone of modern life, used in 75% of household applications.
This steel-cell technique is a very efficient way of generating power from gas and can use the existing gas infrastructure. Overall efficiency of fossil-fuel use can be improved from around 35% to 40% up to 80% to 90%. This means that regular users could reduce the carbon footprint of their home by 30% and even more for the modern always-on business. The combination of these factors makes the steel cell an efficient, cost-effective, and cleaner way of giving people control over their energy supply.
A fuel cell is the most efficient way of converting fuel energy into electricity. It doesn’t matter whether the fuel is natural gas or hydrogen. Fuel cells convert fuel and air directly into power and heat in a chemical reaction. This makes the process efficient, reliable, and quiet.
Fuel passes over the anode side and air passes over the cathode. Sandwiched between the anode and cathode is the very thin electrolyte layer. An external circuit connects the anode to the cathode and provides the mechanism to take power from the fuel cell to power electrical devices.
A single cell can power a low-energy light bulb. Approximately 100 cells are combined to create a stack. One stack could supply up to 90% of a home’s electricity needs and all of its hot water. The steel cell is completely scalable; 200 stacks can supply a large office, apartment block, or supermarket.
FCgen-1020ACS
Ballard Power Systems offers an air-cooled, scalable proton exchange membrane fuel-cell stack suitable for a wide range of light-duty applications where durability, reliability, and a simplified balance of plant are key requirements.
The FCgen-1020ACS fuel cell (Fig. 22-4) has been engineered to incorporate advanced open cathode technology and state of the art self-humidifying membrane electrode assemblies. These features completely eliminate the need for humidification systems and simplify system integration. The result is a simple, low-cost design delivering reliable operation over a wide range of challenging conditions.
22-4. The Ballard Power Systems FCgen-1020ACS has no moving parts and high efficiency, producing clean dc power with a low thermal and acoustic signature.
With no moving parts and high efficiency, the FCgen-1020ACS produces clean dc power with a low thermal and acoustic signature. The FCgen–1020ACS stack can be scaled to meet power requirements from 450W to 3kW and integrated into various end-user applications. The FCgen-1020ACS fuel-cell product is available in a number of cell-configuration options.
Delphi Solid Oxide Fuel-Cell StackEL CELLS
Delphi’s Solid Oxide Fuel Cell (SOFC) technology is commercially ready for a wide range of high-volume stationary power-generation and transportation-industry applications. Delphi’s innovative fuel cell is robust, fuel-flexible, and highly efficient (Fig. 22-5). A single Delphi Gen 4 SOFC Stack can provide 9 kW of electrical power and it features a modular design, ideal for integration into large power plants.
22-5. Delphi’s two stack sizes can be implemented into a stationary or transportation application. Compared to conventional technology, they provide increased efficiency and reliability while decreasing emissions.
Delphi has developed two stack sizes that can be implemented into a stationary or transportation application. They provide increased efficiency and reliability while decreasing emissions when compared to conventional technology. Delphi’s low-cost solid oxide fuel-cell stacks are designed for high-volume manufacturing. Processes are developed and critical suppliers have been identified for all components.
Delphi’s fuel-cell technology can operate with natural gas, hydrogen, gasoline, diesel fuel, bio fuels, or other hydrocarbon fuels. The fuel is converted directly to electrical energy without thermal-mechanical conversion. Therefore, potential efficiency is not limited by the Carnot Cycle and the fuel cell can achieve higher efficiency than internal combustion engines and other conventional power sources. Additional benefits to the fuel-cell technology are the reduction in operating noise and a low level of emissions.
Among other benefits:
• High quality, reliable power:
• Delphi Gen 4 Stack produces up to 9 kW
• Delphi Gen 3 Stack produces 1.5 kW
• Optimum cell sizes (active area) for a range of packaging requirements
• 403}cm3 with the Delphi Gen 4 Stack
• 105cm3 with the Delphi Gen 3 Stack
• Power density at 500mW/cm2
• Stable cell performance
• Equivalent of 40,000 hours of operation
• Delphi has produced more than 30,000 fuel cells
• Thermal Cycle Capability is >200
• High mechanical robustness, achieving the equivalent of 3 million miles in a vibration test schedule.
Read more articles from the Power Management Series in the Power Management section of our Series Library.