Lead-Acid Battery Applications Drive the Li-ion Market
This file type includes high-resolution graphics and schematics when applicable.
The consumer market for lithium-ion (Li-ion) batteries is huge—about $10 billion worth of battery packs—but it’s also relatively flat with only a modest 2% growth rate. Of course, there’s a news-grabbing growth market for electric vehicle batteries, with a forecasted compound annual growth rate (CAGR) of 10%, reaching $10 billion in 2025. Surprisingly, though, the biggest growth area for adoption of new batteries is “everything else,” from forklifts to ventilators. This battery market, which often falls into the “medium format” category, is maintaining a robust 11% CAGR and is also expected to reach $10 billion by 2025.
These “other” applications for Li-ion batteries generally have one thing in common—they’re devices that were typically powered by sealed lead acid (SLA). SLA batteries cornered the portable power market for the last nearly 200 years, but that’s finally changing thanks to Li-ion chemistry formulations with more diverse capabilities and more sophisticated electronics available. Because Li-ion batteries are directly replacing SLA in many cases, it’s worth comparing and contrasting Li-ion and SLA batteries, highlighting the commercial and technical applications for Li-ion in traditional lead-acid products.
History and Application
Lead acid was the first rechargeable battery developed for commercial use in the 1850s. Although lead acid has been around for more than 150 years, it continues to be widely used. Not only is it still found in many of the same applications that it originally enabled, new uses for this old technology are being discovered to this day. Some common applications include backup power or uninterruptible power supply (UPS), and motive applications such as golf carts and forklifts. The lead-acid market is still growing at 4% for niche industries, and projects to be a $1.6B market by 2020 for these applications alone.
The first, and perhaps only, significant innovation to lead-acid technology came in the 1970s, namely sealed lead acid (or maintenance-free lead acid). It includes valves to control venting of gases during charge or rapid discharge. Also, instead of submerging the metal plates in liquid electrolyte, a moistened separator makes it possible to use the battery in any orientation without leakage.
SLA batteries are often categorized by type or application. Two types of SLA technology are common today: gel, also known as valve-regulated lead acid (VRLA), and absorbent glass mat (AGM). AGM batteries are used for small UPS, emergency lighting, and wheelchairs, while VRLA targets larger-format applications such as power backup for cellular repeater towers, internet hubs, and forklifts. Lead-acid batteries can also be categorized by use: automotive (starter or SLI—starting, lighting, ignition); motive power (traction or deep cycle); and stationary (UPS). The major drawback of SLA in all of these applications is cycle life—if the SLA batteries repeatedly drain, they’re heavily damaged.
1. Shown are examples of Li-ion cells incorporated into battery packs with electronics and either hard-plastic enclosures or shrink-wrapping.
Remarkably, the market dominance of lead-acid batteries remained relatively unchallenged for hundreds of years until the introduction of Li-ion batteries in the 1980s. A Li-ion battery is a rechargeable cell in which lithium ions move from the negative electrode to the positive electrode during discharge and back when charging. The nomenclature can be confusing, though. Li-ion batteries use an intercalated lithium compound, but don’t contain metallic lithium, which is used in lithium-based one-time-use cells.
The Li-ion battery was first invented in the 1970s. In the 1980s, a commercial version of the Li-ion battery, with a cobalt-oxide-based cathode, was introduced to the market. This battery featured a significantly better capacity by both weight and volume than nickel-based systems, which had primarily found use in hobby applications and limited adoption in early consumer portable electronics. The new Li-ion batteries enabled enormous growth in cell-phone and laptop computer markets. Initial safety concerns were abated by the introduction of safer variants that incorporated manganese and nickel-based additives into the cobalt-oxide cathode material, in addition to innovations in cell construction.
The first Li-ion cells introduced to the market were in hard aluminum or steel cans, and generally only a handful of cylindrical and prismatic (brick shaped) form-factors were available. However, as more applications adopted Li-ion technology, more variations on the cells emerged in terms of physical form, chemical performance, and price point.
For example, less expensive versions of the older technology lead to the adoption of laptops and cell phones all over the world. Modern, thin Li-polymer cells enabled smartphones, tablets, and wearable electronics. Now high- and medium-rate Li-ion batteries are used in power tools and e-bikes, respectively. Such variation foreshadows the replacement of SLA in more and more applications looking to improve weight and cycle life.
Chemistry Features
The fundamental working chemistry of the cells lends SLA and Li-ion batteries to certain features and different degrees of functionality. What follows are some of the benefits of SLA that have made it the solution of choice for many years and the deficiencies that now threaten it for replacement, as well as the attributes and challenges surrounding Li-ion batteries:
SLA
• SLA is simple, dependable, robust, and inexpensive, and can be used in a wide range of temperature environments.
• The batteries must be stored full state-of-charge (SOC), and they don’t lend themselves to fast charging.
• The flip side to the charge constraints is that SLA batteries can use simple float or trickle chargers.
• SLA batteries are very heavy; their gravimetric energy density is very low.
• Cycle life is usually 200 to 300 cycles, but even a “deep cycle” SLA is damaged by repeated full discharges, causing cycle life to be as low as 50 cycles.
• The sloped discharge curve enables SOC measurement with simple voltage monitoring.
Li-ion
• Conventional Li-ion cells are designed to offer the highest energy density by size and weight.
• Cycle life is generally 300 to 500 cycles, but can be in the thousands for Li-iron phosphate cells.
• The operating temperature is relatively narrow.
• A variety of cell sizes, shapes, and current capabilities are available.
• Maintenance cycling isn’t required and self-discharge is low.
• They require safety circuitry and complex charging algorithms.
• SOC measurement is complex because of the flat voltage discharge curve.
Electronics
It’s important to distinguish between a battery cell and a battery pack. The cell is the fundamental building block of a pack; the pack includes the electronics, connectors, and enclosure. Figure 1 shows some examples of battery cells and packs. At a minimum, a Li-ion battery pack needs to have safety circuitry to protect and manage the cells, and the charger and fuel gauge must be more complex than those used with SLA technology.
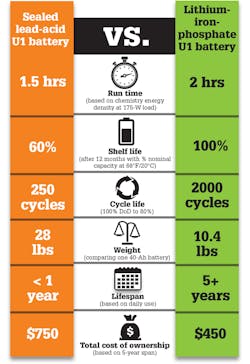
2. U1 replacement batteries have features that contribute to a more efficient model, resulting in significant cost savings over the life of a medical device.
In addition to these issues, the electronics employed in a traditional SLA application will often have special requirements. Generally, these battery packs are “medium format,” i.e., larger than one- or two-cell packs common in consumer electronics and smaller than the enormous batteries used in electric vehicles. In addition, SLA applications are often motive, such as wheelchairs and forklifts, and the motor may introduce system noise and require electronics suited to high power.
When employing Li-ion in an SLA application, some of the differences in the electronics include:
• Charging: Charging a lead-acid battery is simple when observing the correct voltage limits. Li-ion must generally use a more complex algorithm, with the exception of iron-phosphate-based packs (discussed further in the article). The standard method for charging Li-ion batteries is the constant current/constant voltage (CC/CV) method. It incorporates a two-step process of CC followed by a CV charge phase. In the first stage of the charging process, a constant current is applied. This stage continues until the battery-pack voltage approaches the preset threshold at the charger contacts. Once the charging voltage approaches the preset level, current begins to taper exponentially until a cutoff threshold is achieved.
An alternative variation is virtual voltage termination (VVT). By eliminating the effect of resistive losses in the battery pack, the VVT charging algorithm charges the battery-cell stack rather than the outside battery terminals. The charging algorithm therefore doesn’t deviate from the industry-accepted method for Li-ion battery packs. With VVT, the CC stage extends for most of the charging period, until the microprocessor senses nominal cell voltage and initiates the transition event.
• Fuel gauging and communication: As mentioned earlier, SLA batteries are generally monitored with a simple voltage measurement. Li-ion batteries require a coulomb-counting method, which requires learn cycles or a variation of cycles, or a combination of coulomb counting and impedance lookup tables to avoid the learn cycles.
I2C is the most common and cost-effective communications protocol used in Li-ion batteries, but it has limitations with respect to noise immunity, signal integrity over distance, and overall bandwidth. SMBus (System Management Bus), a derivative of I2C, is very common in smaller batteries, but currently lacks any particularly effective support for high-power packs or larger packs. CAN is terrific for high-noise environments or where long runs are needed, such as in many SLA applications, but it comes at a cost.
Direct Replacements
Surprisingly, there are few standard SLA formats. One of them is the U1, a common standard form factor used in medical-equipment backup applications. Li-iron phosphate chemistry has enabled the development of direct drop-in replacements for these batteries. Iron phosphate features remarkable cycle life, high-current delivery capability, increased safety, and low impedance. The voltage of Li-iron-phosphate batteries also matches well with SLA at 12- and 24-V increments, and allow for use with conventional SLA chargers. Packs incorporate smart features such as state-of-charge and cycle-count communication at about one third the weight.
Li-iron-phosphate batteries maintain 100% capacity in storage, unlike SLA batteries that experience permanent capacity loss within a few months of storage. Figure 2 compares the two products and the types of gains achieved when switching from SLA to Li-ion.
Conclusion
Few other batteries can deliver bulk power as cheaply as lead acid, which makes the battery cost-effective for larger applications. Li-ion technology is coming down in price point and new varieties are available in both chemistry and electronics that make Li-ion a potential replacement for SLA, with significant upside in cycle life and weight. Applications ripe to take advantage of this technology include material-handling equipment, battery backup units, lawn and garden equipment, industrial cleaners, aerial scissor lifts, industrial drones, golf carts, and other motive applications, which are fueling Li-ion’s growth in niche, medium-format markets.
Robin Schneider, Ph.D., is Technical Marketing Director at Inventus Power.