Stimulated Raman Microscopy Provides Battery Ion-Transport Insight
Batteries present a major difficulty from an R&D perspective. Because they’re mostly “black boxes,” it’s very difficult to observe what’s going on inside of them while they’re functioning, and a destructive autopsy is far different and less insightful than live exploration. However, a team based at Columbia University has contributed a major solution to this shortcoming by using Raman-scattering microscopy, an advanced spectroscopic electro-optical technique widely used in biomedical testing, chemical-process investigation, and materials science.
Their paper “Operando and three-dimensional visualization of anion depletion and lithium growth by Stimulated Raman microscopy,” published in Nature Communications, describes in great detail how they used this approach to investigate a long-standing issue: How does the concentration of lithium ions in a battery cell relate to uneven lithium deposition? Visualization of the ion transport is of great interest because it can provide deep insight into the dynamics of the electrolyte movement and transformations. Furthermore, they were able to develop new understanding and even arrive at likely conclusions based on their arrangement.
In theory, using pure lithium for a battery electrode will improve its efficiency, but the nonuniform flux of ions near that electrode is detrimental, encouraging nucleation and growth of detrimental dendrite-like crystals that reduce efficiency (and may even cause dangerous short circuits). The research challenge is to measure the growth of these crystals by imaging the movement of the ions themselves, but this is a very difficult goal due to their low concentration and fast reaction times.
Stimulated Raman Scattering
That’s where stimulated Raman scattering (SRS) offers a new approach. In non-stimulated, spontaneous technique, the energy of spontaneous photon scattering of the target molecule is measured, thus revealing its natural vibration frequency (see figure). (Note: to avoid confusion, spontaneous Raman scattering is not called SRS.) In the stimulated scattering variation, a synchronized pump-laser beam and a swept-wavelength Stokes laser beam are combined, and that interacts with the sample, which causes it to generate a coherent optical response.
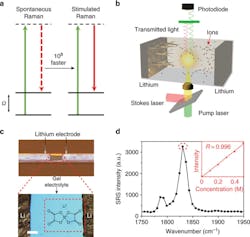
In spontaneous Raman scattering, only one laser (green, shown by solid line) is used; in SRS, two different lasers (green/red, solid line) are simultaneously used and yield up to 108 times faster vibrational transitions (a). In the schematic illustration of a Li–Li symmetric cell under SRS imaging, the two lasers are the pump laser and Stokes laser (b). In the image of a Li–Li symmetric cell (top) and zoom-in microscope image (bottom), the red, dashed rectangle indicates the imaging area, while the molecular structure is that of the lithium bis(oxalato)borate (LiBOB) salt used in the study (c). And in the SRS spectrum of the gel electrolyte, the inset shows the linear concentration dependence between the Raman intensity at 1830 cm–1 (dashed circle) and the LiBOB concentration from 0 M to 0.5 M (d).
By measuring the vibrational resonances as the impinging beam is swept, the detailed “fingerprints” of the target molecules are revealed. The stimulated Raman scattering setup that was used accelerated the fairly slow speed of standard spontaneous Raman scattering by eight orders of magnitude. It combined high sensitivity of better than 0.5 millimoles (mM) with fast imaging speed of about 2 µs/pixel and resolution of 300-500 nm.
Research Results
In the experiment, the research team measured the intensity of anion response, which functions as an “analog” for the lithium-ion concentration, as they have similar concentration to the lithium cations (a difference of less than 0.1 mM) and bond with them. The results revealed a multi-stage positive-feedback mechanism resulting in uneven growth of ion concentration. Those regions that had a higher concentration underwent a higher growth rate, which in turn led to further increase in concentration and accelerated growth rate of the undesirable dendrite crystals.
This insight also led them to develop a possible approach to mitigate the problem. The researchers developed a coating of lithium phosphate (Li3PO4) acting as an artificial solid electrolyte interphase, which was tough enough to suppress the undesired protrusions on the electrodes. It did this by functioning as a chemical buffer, which made the local Li-ion concentration more homogenous and blocked the positive-feedback loop, thus decreasing the crystal growth rate without affecting the voltage charge/discharge profile.
Beyond the analysis results and insight, the researchers demonstrated that this stimulated Raman technique provides a valuable and viable imaging tool. It can be used to investigate ion transport and processes in battery research, and even be extended to observing material separation, the dynamics of ion movement in confined channels, and associated catalytic processes.