Improved Chemistry Enhances Zinc-Battery Recharge, Safety, Performance
There’s nothing new about use of zinc in battery cells; it was first employed in 1799. However, lithium-based cells have become the preferred choice due to their favorable energy density with respect to both mass and volume—as well as their excellent recharge capability—despite the well-known safety issues associated with lithium chemistries. Zinc batteries are still used in applications which do not demand the highest energy-density performance, where rechargeability is not crucial, or where safety is a major issue.
To overcome these limitations, a joint team from the U.S. Army Research Laboratory (ARL), Adelphi, Md.; the University of Maryland (UMD), College Park, Md.; and the National Institute of Standards and Technology (NIST) Center for Neutron Research, Gaithersburg, Md. focused on improving the basic performance of zinc chemistry—especially in the recharge cycle. They have devised and tested a new class of water-based electrolytes, with results published in Nature (“Highly reversible zinc metal anode for aqueous batteries”) along with detailed supplementary information. Their aqueous (water-based) zinc-battery solution has relatively high capacity, is rechargeable, and is intrinsically safe.
In principle, metallic zinc (Zn) is a good anode material for aqueous batteries due to its high theoretical capacity (820 mA-hr/gm), low potential (−0.762 V measured with respect to the standard hydrogen electrode), abundance in nature and ease of mining, low toxicity, and intrinsic safety. However, there are also some attributes of zinc batteries that have worked against them:
- They exhibit poor reversibility of their chemistry in aqueous electrolytes, meaning they do not recharge well, due to low coulombic efficiency (CE, also known as Faraday efficiency, is the ratio of the total charge extracted from the battery to the total charge put into the battery over a full cycle);
- Their dendrite growth during elementary plating/stripping of the discharge/recharge cycle will eventually short out the cell ((dendrites are metallic finger-like growths);
- Their ongoing water consumption means they require maintenance and refilling.
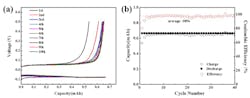
The team developed zinc-batteries with both LiMn2O4 and LiMn2O2 cathodes, with the former delivered180 W-hr/kg while retaining 80% capacity for ore than 4,000 cycles, and the latter delivered 300 W-hr/kg for more than 200 cycles (see figure). Their approach uses a water-in-salt aqueous electrolyte based on zinc and lithium salts at high concentrations, which allowed for dendrite-free zinc plating/stripping at nearly 100% CE. The water molecules in the electrolyte are strongly bonded by the highly concentrated salt, so the water in the electrolyte will not evaporate even in an open cell—thus enabling maintenance-free batteries.
Shown are a) the voltage profiles of Ti/Zn coin cell in the first 10 cycles; and (b) the corresponding columbic efficiency of zinc metal, both during the zinc-metal plating/stripping cycle under conditions specified in the paper’s supplementary information. (Image source: U.S. Army Research Laboratory and University of Maryland)
Chunsheng Wang, a UMD professor of chemical and biomolecular engineering and a corresponding author of the paper, notes that “existing zinc batteries are safe and relatively inexpensive to produce, but they aren't perfect due to poor cycle life and low energy density. We overcome these challenges by using a water-in-salt electrolyte.”
ARL fellow and team leader Dr. Kang Xu added that “the safety hazard of lithium-ion batteries are rooted in the highly flammable and toxic non-aqueous electrolytes used therein. The batteries of aqueous nature thus become attractive, if they can be made rechargeable with high energy densities. Zinc is a natural candidate.”
The research received funding support from the Department of Energy Advanced Research Projects Agency-Energy and the University of Maryland Center for Research in Extreme Batteries.