When Tesla Motors revealed its plan to buy solar panel maker SolarCity, the thread tying them together was battery storage. The idea, according to chief executive Elon Musk, is to combine solar panels with Tesla’s stationary Powerwall batteries. On cloudy days or in the evening, the stored energy could run your dishwasher or charge your Tesla Model S sedan.
For the batteries inside its cars and Powerwall systems, Tesla is betting on the same lithium-ion chemistry used in smartphones and hover-boards. Other companies and researchers think there is a better option. Many are experimenting with new lithium-air batteries that are smaller, lighter, and more energy-efficient than their forebears.
On Monday, scientists from the Massachusetts Institute of Technology, the Argonne National Laboratory, and Peking University in China revealed a promising new version of lithium-air batteries. Their new device, the scientists said, can serve as a drop-in replacement for lithium-ion, while storing over five times more energy as today’s batteries.
“This means faster charging for cars, as heat removal from the battery pack is less of a safety concern, as well as energy efficiency benefits,” said Ju Li, an MIT professor of nuclear science and engineering and author of the research.
The new device, which was described in the journal Nature Energy, is the latest vying to become the replacement for lithium-ion. Scientists from IBM and the startup PolyPlus are placing bets on lithium-air, while other companies are working toward zinc-air and sodium-air batteries.
The new design “has added a further promising high energy storage battery system into the mix,” wrote Lawrence Hardwick, a chemist at the University of Liverpool, in a Nature article about the research. But he admitted that “as yet, no obvious front-runner has emerged.”
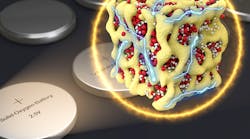
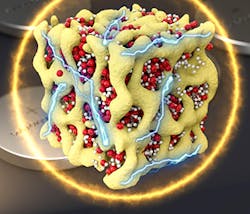
Holding back lithium-air batteries has been their chemistry. Until now, lithium-air batteries inhaled outside air—driving a chemical reaction with the battery’s lithium—while electric current flows out. This oxygen is released to the atmosphere during the charging cycle. The chemical reaction produces other molecules, known as lithium peroxide, that slowly clog the battery electrodes.
That raises several problems. According to Li, the solid particles formed by the reaction cause the battery to degrade faster than lithium-ion batteries, which are completely sealed from outside air. When the battery degrades, it stores less energy.
The battery is also prone to losing energy in the form of heat. Its output is more than 1.2 volts lower than the voltage needed to charge it, causing it to lose 30% of the electricity as heat. Because of that, the battery can “actually burn if you charge it too fast,” said Li. Overcharging can lead to structural damage or an explosive reaction known as thermal runaway.
The new design solves these problems by closing off the battery to outside oxygen. The same electrochemical reactions take place between lithium and oxygen during charging and discharging, but they take place without ever using oxygen gas. Instead, the oxygen stays inside the battery and switches between three solid chemical compounds: Li2O, Li2O2, and LiO2. This prevents the damaging particles from forming.
The new battery was paved by an earlier study in lithium-air chemistry. Earlier this year, two of the engineers involved in the new report—Khalil Amine and Jun Lu from the Argonne National Laboratory—helped create the lithium superoxide LiO2, while the battery was discharging. The compound can more easily split into its parts than lithium peroxide, making it more efficient and longer-lasting.
“This discovery really opens a pathway for the potential development of a new kind of battery,” Larry Curtiss, an Argonne National Laboratory chemist, said about the experiment in January. “Although a lot more research is needed, the cycle life of the battery is what we were looking for.”
The new battery sharply cuts voltage loss, so only 8% of the electrical energy is lost as heat. It also inherently guards against overcharging: The device can shift between different lithium compounds if it is being overcharged to stop activity that might cause damage. The scientists overcharged the battery to 100 times its capacity for 15 days without any damage. They also found that through 120 charging cycles, the battery only lost 2% of its capacity.
The lithium-air battery can also do without components to pump air inside and out of the battery. Without these auxiliary parts, it can easily be adapted to existing devices or battery packs inside cars and power grid storage.
For now, the researchers are keeping to the laboratory. The team expects to make a practical prototype of the device within about a year.