Freezing Plus Phase Change May Yield Safer, Flexible Lithium Batteries
The dangers associated with lithium-based batteries are well-known to designers. Any inconsistencies in the manufacturing process, mismanagement during charging/discharging cycles, or improperly managed thermal issues can cause fire and even explosion. It comes as no surprise, then, that the search for a safer way to build these high-density, lightweight, electrochemical energy-storage components has attracted significant attention.
A four-person team at Columbia University’s Fu Foundation School of Engineering and Applied Science developed a technique that may offer a viable approach to a better electrolyte and, by extension, batteries.1,2 By controlling the structure of the solid lithium electrolyte, they developed a solid electrolyte that’s safer, non-flammable, and non-toxic, thus avoiding the concerns associated with liquid electrolytes. At the same time, this process may result in a battery that’s bendable to more easily fit the available enclosed space—and one with a longer lifecycle.
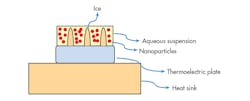
1. In ice templating, a thermoelectric plate is used to cool the solution to an icy state. Then, a vacuum is applied that induces sublimation with a direct phase transition to gaseous state, while the desired ceramic electrolyte material remains. (Source: ACS Publications)
Creation of the electrolyte is based on lithium-aluminum-titanium-phosphate Li1+xAlxTi2-x(PO4)3 nanoparticles (LATP NPs), which are processed with other chemicals to form a ceramic precipitate. Normally, the high conductivity of ceramic fillers is compromised to a large extent by the low conductivity of the resultant matrix, especially when nanoparticles are used. Here, an ice-templating process is employed (Fig. 1), in which an aqueous solution with the ceramic particles is cooled from the bottom up (starting at the top of a thermoelectric plate). The cooling rate is controlled by a LabView program.
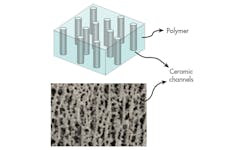
Next, a vacuum is applied to the ice, forcing its direct transition to a gas state (sublimation), while leaving a vertically aligned structure; going directly without an intervening liquid phase minimizes contamination of the structure. Finally, this ceramic structure is combined with a polymer to provide mechanical support and flexibility to the electrolyte. The vertically aligned and connected LATP NPs in the polyethylene oxide (PEO) matrix maximize the ionic conduction, while maintaining the flexibility of the composite.
The conductivity of this vertically aligned structure (Fig. 2) reaches 0.52 × 10–4 S/cm, which is about 3.6 times higher than that of a composite electrolyte with randomly dispersed LATP NPs. The composite electrolyte also shows improved thermal and electrochemical stability compared to the pure PEO electrolyte. [Conductivity (siemens/cm) is measured using the standard electrochemical impedance spectroscopy (EIS) technique.3,4]
2. A schematic of the ceramic-based polymer electrolyte and a microphotograph show that the resultant material matrix is straight, which enables faster ion transport and thus increased conductivity. (Source: ACS Publications)
Researchers in earlier studies used either randomly dispersed ceramic particles in polymer electrolyte or fiber-like ceramic electrolytes that were not vertically aligned, and the randomly dispersed ceramic particles in the polymer matrix blocked the ion transport.
“We thought that if we combined the vertically aligned structure of the ceramic electrolyte with the polymer electrolyte, we would be able to provide a fast highway for lithium ions and thus enhance the conductivity,” says PhD student Haowei Zhai, the paper’s lead author. He adds, “We believe this is the first time anyone has used the ice-templating method to make flexible solid electrolyte, which is nonflammable and nontoxic, in lithium batteries.”
In addition, this technique could in principle enhance the energy density of batteries. By using the solid electrolyte, the lithium battery’s negative electrode, which is currently a graphite layer, could be replaced by lithium metal, and thus could improve the battery’s specific energy by 60% to 70%.
Student Zhai and project leader Yuan Yang, an assistant professor of materials science and engineering, next plan to try to optimize the attributes of the combined electrolyte, and then assemble the flexible solid electrolyte with battery electrodes to construct a prototype of a complete lithium battery.
References
1. ACS Publications Letters, “A Flexible Solid Composite Electrolyte with Vertically Aligned and Connected Ion-Conducting Nanoparticles for Lithium Batteries”
2. ACS Publications, “Supporting Information: A Flexible Solid Composite Electrolyte with Vertically Aligned and Connected Ion-Conducting Nanoparticles for Lithium Batteries”
3. Gamry Instruments, “Basics of Electrochemical Impedance Spectroscopy”
4. University of Connecticut, “Basics of Electrochemical Impedance Spectroscopy”