Lithium-based cells are the chemistry of choice for many battery applications due to their favorable power/weight and power/volume ratios, but also have a drawback with the well-known risks of fire and even explosion. Another element, magnesium is a potential alternative, but it has issues with its cathode’s performance and electrolyte efficiency.
Now, a joint team from three universities and three national laboratories, led by researchers at the University of Houston, reports they have developed a magnesium-based cathode design that counters conventional wisdom about the critical magnesium-chloride bonds. The results were reported in Nature Communications (the posted abstract includes a read-only link to the full paper). The new battery has storage capacity of 400 mA-hr/gm, as opposed to the 100 mA-hr/gm for earlier magnesium batteries.
Yan Yao, associate professor of electrical and computer engineering at the University of Houston and lead author on the paper, pointed out that the “magnesium ion is known to be hard to insert into a host. First of all, it is very difficult to break magnesium-chloride bonds. More than that, magnesium ions produced in that way move extremely slowly in the host. That altogether lowers the battery’s efficiency.”
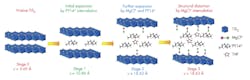
The structural evolution of titanium disulfide at different stages of intercalation shows how interlayers are expanded or distorted as different amounts of pillaring molecules, complex cations and solvents are intercalated into the van der Waals gap of a host material at each stage. (Source: University of Houston)
The team combined a nanostructured cathode and a new understanding of the magnesium electrolyte. Their approach stores energy by inserting magnesium monochloride into a host such as titanium disulfide. By retaining the magnesium-chloride bond, said Yao, the cathode demonstrated much faster diffusion than traditional magnesium versions.
They accomplished this by expanding the titanium disulfide to allow magnesium chloride to be inserted in a four-step process called intercalation, rather than break the magnesium-chloride bonds and insert the magnesium alone. However, implementing the intercalation process isn’t easy, as the magnesium monochloride molecules are too large to be directly inserted into the titanium disulfide using standard methods.
Therefore, the researchers instead created an open nanostructure by expanding the gaps in the titanium disulfide by a factor of three, using organic “pillars” (see figure). Although the opening was still small, going from 0.57 nm to 1.8 nm, that was enough to allow the magnesium chloride to be inserted.
Terminal voltage of cells based on the new approach is low—about 1 V—while lithium-based cells produce 2 to 4 V. The new battery’s storage capacity of 400 mA-hr/gm compares very favorably to commercial lithium-ion batteries with their cathode capacity of about 200 mA-hr/gm, said Prof. Yao, who is also a principal investigator with the Texas Center for Superconductivity at UH. (Of course, there’s often a significant gap between battery performance in the lab versus mass-production reality.)
“We hope this is a general strategy,” added Yao. “Inserting various polyatomic ions in higher voltage hosts, we eventually aim to create higher-energy batteries at a lower price, especially for electric vehicles.”