Lithium-based chemistries dominate today’s battery technologies due to their high energy density by volume and by weight. But they may not be the only option, as researchers at Caltech, the Jet Propulsion Laboratory, the Honda Research Institute, and Lawrence Berkeley National Laboratory and others are working together to develop rechargeable batteries based on fluoride, the anion (negatively charged form) of elemental fluorine.
Why fluorides? Theoretical energy density is determined largely by the number of electrons transferred in the electrochemical reaction, the cell voltage (potential difference) between cathode and anode, Faraday’s constant, and the cell volume—areas where fluorides have some attractive characteristics.
The fluoride ions used in the new study bear a negative charge (anions), while lithium ions are positive (cations). "For a battery that lasts longer, you need to move a greater number of charges. Moving multiple charged metal cations is difficult, but a similar result can be achieved by moving several singly charged anions, which travel with comparative ease," noted Simon Jones, a chemist at JPL involved in the project. The low atomic weight of fluorine could result in batteries with very high energy density.
The BTFE Effect
However, such batteries need to operate at impractically high temperatures due to their molten salt electrolytes, which are solid at moderate temperatures. The researchers developed a fluoride-based, ion-conducting electrolyte with high ionic conductivity, wide operating voltage, and robust chemical stability using a liquid called bis(2,2,2-trifluoroethyl)ether, or BTFE. This solvent keeps the fluoride ion stable so that it can move electrons back and forth in the battery.
Once they had the basic BTFE solution worked out, they ran computer simulations of the reaction to determine which aspects of BTFE were stabilizing the fluoride. They were then able to slightly modify the BTFE solution to improve its performance.
Study co-author Robert Grubbs, the Victor and Elizabeth Atkins Professor of Chemistry at Caltech and a winner of the 2005 Nobel Prize in Chemistry, noted that "fluoride batteries can have a higher energy density, which means that they may last longer—up to eight times longer than batteries in use today. But fluoride can be challenging to work with, in particular because it's so corrosive and reactive."
[Interesting side note: Uranium hexafluoride—UF6—is a gaseous compound used in enriching uranium to produce fuel for nuclear reactors and weapons.. It’s highly toxic, reacts with water, and is corrosive to most metals. Developing radically new pump designs that could handle the UF6 gas in centrifugal isotope separation was a significant design challenge for the Oak Ridge (TN) facility producing material for the atomic bombs in World War II, see here.]
Protecting the Core
A suitable electrolyte is only part of the fluoride-based battery story. To manage the challenges associated with cathode-metal dissolution, they designed composite cathode materials featuring a core-shell nanostructure with an inert thin shell around the active material as an artificial solid-electrolyte interphase (SEI). This served multiple objectives: It protected the active core from dissolution; protected the electrolyte from decomposition; restricted volume expansion and so maintained structural integrity of the core; and selectively percolated electrolyte ions into the core.
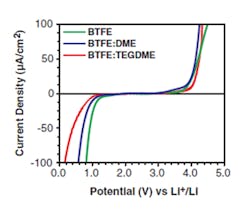
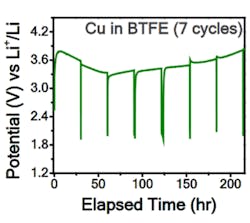
The research team has demonstrated reversible reactions in a fluoride-ion electrochemical cell that could be cycled (charged/discharged) at room temperature. To better understand what was happening within the cell, they needed to apply highly advanced measurement techniques. One was electron energy-loss spectroscopy (EELS) using scanning transmission electron microscopy (TEM), where a beam of electrons is transmitted through a specimen to form an image. They also used X-ray photoelectron spectroscopy (XPS) depth-profile studies of thin-film structures to confirm their assessments of fluoride-ion transport and diffusion. Among their many detailed tables and graphs are two showing current density and potential compared to lithium cells (Figs. 1 and 2).
1. Fluoride-based cell-current density versus lithium chemistry for various BTFE formulations. (Source: Caltech)
2. The room-temperature voltage profile using a copper cathode in a three-electrode cell was close to the expected value, but the reasons for discrepancy are unclear and still speculative. (Source: Caltech)
Their comprehensive paper in Science, “Room-temperature cycling of metal fluoride electrodes: Liquid electrolytes for high-energy fluoride ion cells,”plus their exhaustive Supplementary Materials, provide full electrochemical details. Their hope is that the dual advances of a room-temperature fluoride-liquid electrolyte as well a copper-lanthanum trifluoride core-shell cathode material may yield batteries with higher energy density than lithium-based devices.
Of course, many battery advances have been demonstrated in the lab, but ones that actually become practical and successful are rare for many reasons (cost, production issues, consistency, reliability, application-related shortcomings, among others). However, with so many independent R&D efforts underway, the law of large numbers may produce a winner that enhances or surpasses today’s lithium-based cells.