>> Website Resources
.. >> Library: TechXchange
.. .. >> TechXchange: Power Management
.. .. .. >> Topic: Energy Harvesting
When we think of rust—iron oxide—we usually refer to its negative effects in terms of corrosion and material degradation. But rust does have its uses: It can function in some cases as a protective coating, and now perhaps as a source of harvested electrical power. A team of researchers at Northwestern University (with some support from Caltech) has devised a rust coating that can be used to generate electricity via the flow of saltwater, converting the kinetic energy of flowing droplets and, thus, alternating salinity gradients.
This arrangement isn’t a more-sophisticated version of the classic school-science demonstration where dissimilar metal contacts, inserted into a lemon or potato, generate a small current using the internal liquid as the electrolyte. Here, the ions present in saltwater attract electrons in the iron beneath the layer of rust. As the saltwater flows, the ions attract, dragging along the electrons and generating an electrical current.
This "electrokinetic" effect has already been used with graphene, the ultra-thin layers of pure carbon that are finding diverse application. However, scaling the single-layer graphene up to usable sizes is a challenge, and the researchers note that their iron-oxide films are easier to produce.
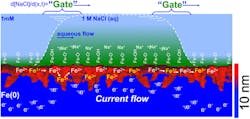
Even so, this isn’t a randomly grown rust coating, since what’s needed is a consistently thin layer. To achieve this, the team used a process of physical vapor deposition, where they first transformed solid iron into a gas and then condensed that vapor onto a glass surface, creating an iron layer 10 to 30 nm thick. An oxide layer then forms spontaneously in air to a thickness of 2 nm and subsequently stops growing (Fig. 1).
1. Graphical representation of electrical energy conversion in metal nanolayers terminated by their thermal oxides. (Source: Northwestern University)
“It’s the oxide layer over the nanometal that really makes this device go,” said project leader Franz M. Geiger, the Dow Professor of Chemistry in Northwestern’s Weinberg College of Arts and Sciences. “Instead of corrosion, the presence of the oxides on the right metals leads to a mechanism that shuttles electrons.”
He also noted that “thicker films of metal don’t succeed—it’s a nano-confinement effect…we have discovered the sweet spot.” Of the metals studied, the researchers found that iron, nickel, and vanadium worked best. They even tested a pure rust sample as a control experiment, but it didn’t produce a current.
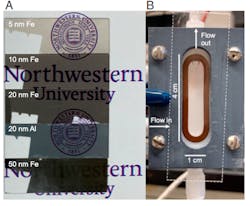
2. Metal nanolayers for gravitational to electrical energy conversion: Photographs of iron and aluminum nanolayers with indicated thicknesses on microscope glass slides over the Northwestern University seal (A); photograph of Teflon cell with flow channel (B). (The dashed lines indicate substrate position and arrows indicate aqueous flow direction.) (Source: Northwestern University)
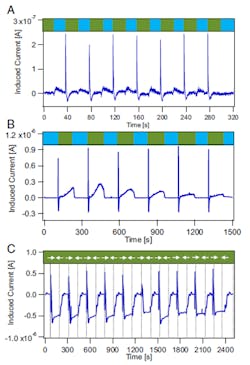
They were able to produce potentials of several tens of millivolts and current densities of several µA/cm2 at flow velocities of just a few cm/sec in their test fixture (Fig. 2). They tested both a linear flow with salinity gradients as well as an oscillatory flow of a constant salinity (Fig. 3).
3. Current and voltage measurements: Current induced in a 10-nm Fe:FeOx nanolayer (3 × 1 in) when flowing deionized (DI) water at pH 5.8 for 20 sec (blue segment), followed by 20-sec flow of 1 M NaCl held at pH 7 (green segment), and six subsequent replicates, all at a constant flow rate of 20 mL/min (A). The (B) plot is the same as in (A), but measured using a 3- × 9-in Fe:FeOx nanolayer of 10-nm thickness at a flow rate of 100 mL/min and two minutes between switching salt concentration The (C) plot is the same as in (B), but measured at a flow rate of 35 mL/min and constant 0.6 M salt concentration while reversing the flow direction every two minutes, marked by the vertical dashed lines. (Source: Northwestern University)
Of course, this being energy conversion, there is no “something for nothing.” Work in the physics sense must be done and energy expended to create that flow of saline solution, unless it comes from freely occurring sources such as rainfall or ocean currents. Efficiency is claimed to be around 30% based on theoretical analysis and simulation versus actual results. It’s similar to results achieved with other materials, such as carbon nanotubes and graphene. However, it employs a far easier, lower-cost, single-step fabrication that also supports scalability and rapid implementation instead of multistep fabrication. Northwestern has filed for a provisional patent.
The researchers maintain that a surface of 10 square meters (on a roof, for example) could generate a few kilowatt-hours with suitable flow, such as from rainfall. On a much-smaller scale, Prof. Geiger remarked that a stent coated with the metal nanolayers might produce a microampere or so due to intermittent blood flow with each heartbeat. With one hundred thousand such beats per day (100,000), that’s about 0.1 A/day that can be stored in a capacitor to drive an implanted device (such as an insulin pump) on demand. At the same time, team member Tom Miller (a Caltech professor of chemistry) cautioned that they would realistically have to contend with the formation of biofilms on the surfaces that could damp the electricity-conversion effect.
Full details are available in their paper “Energy conversion via metal nanolayers” published in the Proceedings of the National Academy of Sciences; there’s also detailed Supporting Information posted. This work was supported by the National Science Foundation, the Office of Naval Research, and the Defense Advanced Research Projects Agency (DARPA) through the Army Research Chemical Sciences Division.
>> Website Resources
.. >> Library: TechXchange
.. .. >> TechXchange: Power Management
.. .. .. >> Topic: Energy Harvesting