The quest for better lithium-based batteries continues with signs of progress but no long-term guarantees. Certainly, even a small improvement in capacity, charger time, or other performance attribute would be significant, given the unit volume of these batteries in present and anticipated uses ranging from small multicell packs to the larger assemblies used in electric vehicles and hybrid EVs. It would even benefit those fixed-in-place trailers and warehouse-size installations that are intended to store excess wind or solar power for later use.
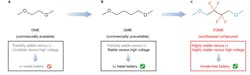
At Stanford University, a team is looking to improve the electrolyte chemistry so that lithium-metal (Li-M) batteries can be used in place of lithium-ion (Li-ion) devices. As their name suggests, these batteries use a lithium-metal anode instead of a graphite anode and have about twice the potential energy density per kilogram as today’s conventional Li-ion batteries.
However, study co-author Zhenan Bao, the K.K. Lee Professor in the School of Engineering, noted that Li-M has a problem. She says, “…during operation, the lithium-metal anode reacts with the liquid electrolyte. This causes the growth of lithium microstructures called dendrites on the surface of the anode, which can cause the battery to catch fire and fail.”
To overcome this significant problem, the team focused on organic chemistry to principles of design and create new, stable electrolytes for these batteries. Zhiao Yu, a graduate student in chemistry and co-lead author of the published paper, remarked “We hypothesized that adding fluorine atoms onto the electrolyte molecule would make the liquid more stable. Fluorine is a widely used element in electrolytes for lithium batteries. We used its ability to attract electrons to create a new molecule that allows the lithium-metal anode to function well in the electrolyte.”
The result was a novel synthetic compound, abbreviated FDMB, that can be readily produced in bulk (see figure). In addition to longer cycle life and better stability, the FDMB electrolyte is also far less flammable than conventional electrolytes.
With batteries, of course, the characterization of any improvement is defined by standard tests. The experimental battery retained 90% of its initial charge after 420 cycles of charging and discharging, compared to typical lithium-metal batteries that stop working after about 30 cycles. The researchers also measured coulombic efficiency, which characterizes how efficiently lithium ions are transferred between the anode and the cathode during charging and discharging.
Study co-author Yi Cui, professor of materials science and engineering and of photon science at the SLAC National Accelerator Laboratory (formerly called the Stanford Linear Accelerator Center), said, “To be commercially viable, a battery cell needs a coulombic efficiency of at least 99.9%… we got 99.52% in the half cells and 99.98% in the full cells; an incredible performance.”
More details are in their paper “Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries” published in Nature, along with extensive additional tables and analysis in the Supplementary Information file.
Also, check out this very short video with a flammability test of conventional carbonate electrolyte (left) and the novel FDMB electrolyte (right) developed at Stanford:
The conventional carbonate electrolyte is flammable immediately after touching the flame, but the FDMB electrolyte can tolerate the direct flame for at least three seconds.
“The anode-free battery in our lab achieved about 325 watt-hours per kilogram specific energy, a respectable number,” said Cui.
This study was supported in part by a grant from the Battery500 research consortium that includes the U.S. Department of Energy, Stanford, and SLAC. Its objective is to make lithium-metal batteries viable. The consortium’s goals are to nearly triple the amount of energy that a lithium-metal battery can deliver from about 180 watt-hours per kilogram when the program started in 2016 to 500 watt-hours per kilogram.