Novel Electrolyte May Enable Manufacturers to Easily Boost Li-Ion Battery Life, Capacity
What you'll learn:
- What materials are used in the new electrolyte?
- How will it impact manufacturers with their current products?
- Does it address the cracking problem?
- Results achieved with the new electrolyte to this point.
A team of researchers, led by MIT, announced a new electrolyte for lithium-ion batteries that could allow manufacturers to improve their existing products' energy densities by as much as 60%. Once commercialized, manufacturers should be able to use their existing equipment to produce affordable products that significantly extend the range of electric vehicles, make electric aviation more practical, and accelerate the de-carbonization of the world's economy. The research was supported by the U.S. Department of Energy and the National Science Foundation, and made use of facilities at Brookhaven National Laboratory and Argonne National Laboratory.
The new electrolyte, originally developed for use in experimental lithium-air batteries, is expected to make it possible to produce Li-Ion cells with metallic electrodes using the same equipment currently used to make today's cells that use graphite-based electrodes. The nickel-based electrodes in the team's experimental batteries can be charged to 4.7 V, significantly higher than what's possible with today's Li-ion products.
As a result, manufacturers could dramatically increase the energy density of their existing products from today's average of 240-260 Wh/kg to as much as 420 Wh/kg. If successfully commercialized, these improvements would make it economically feasible to offer longer ranges for EVs, longer-lasting charges on portable devices, and enable other advances that would accelerate the de-carbonization of the global economy. (For example, referring to image above, the novel electrolyte could enable manufacturers to use their current equipment to produce batteries that give vehicles like this BMW i3 as much as 60% more range; credit: Rudolf Simon.)
Battery researchers have long known that replacing conventional batteries' graphite electrodes with metallic ones could produce a higher charging voltage in the cathode. However, until now, efforts to use them in a practical commercial product have been hampered by the chemistry used in nearly all of today's lithium-based cells.
Researchers at MIT, the Oak Ridge Institute for Science and Education, and elsewhere discovered a novel electrolyte that overcomes several unwanted chemical reactions that occur with the electrolyte separating the electrodes. The research was reported in the journal Nature Energy in a paper by MIT professors Ju Li, Yang Shao-Horn, and Jeremiah Johnson, postdoc Weijiang Xue, and 19 others at MIT, two national laboratories, and elsewhere.1
Electrolyte Materials
The researchers say that the new chemistry can be utilized to modify the process currently used to produce commercial Li-ion batteries. It doesn’t require manufacturers to design a completely new battery, or radically alter their manufacturing lines. The basic raw materials for this electrolyte are inexpensive (though one of the intermediate compounds is still costly because it’s in limited use), and the process to make it is simple. Thus, this advance could be implemented relatively quickly, the researchers say.
The new application of this electrode material was found “somewhat serendipitously,” explains Johnson, a professor of chemistry. It was developed a few years ago by by Shao-Horn, Johnson, and others, in a collaborative effort to create commercially viable lithium-air batteries, which are seen as the ultimate long-term solution for maximizing battery energy density. But many obstacles still face the development of such batteries, and that technology may still be years away. In the meantime, the team found that applying the electrolyte to lithium-ion batteries with metal electrodes can provide an excellent interim solution.
“There's still really nothing that allows a good rechargeable lithium-air battery,” says Johnson. However, “we designed these organic molecules that we hoped might confer stability, compared to the existing liquid electrolytes that are used.”
They developed three different sulfonamide-based formulations, which they found were quite resistant to oxidation and other degradation effects. Then, working with Li’s group, postdoc Xue decided to try this material with more standard cathodes instead.
Their current experiments center around using the new electrolyte with electrodes composed primarily of nickel oxide, with smaller amounts of cobalt and manganese added. "This is the workhorse of today’s electric-vehicle industry,” says Li, who is a professor of nuclear science and engineering and materials science and engineering.
Attacking the Cracking
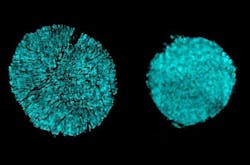
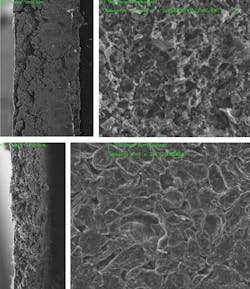
The problem has been that, when used with conventional electrolytes, nickel-based electrodes work well initially but degrade a battery's performance over time. This is due to cracking that occurs as the electrode material expands and contracts anisotropically during charge and discharge cycles. But in experiments in collaboration with Brookhaven National Laboratory, the researchers found that using the new electrolyte drastically reduced these stress-corrosion cracking degradations.
They discovered the cracking was occurring because the metal atoms in the alloy tended to dissolve into the liquid electrolyte, causing erosion that lead to cracks in the metal. In contrast, the new electrolyte is extremely resistant to such dissolution. Looking at the data from the Brookhaven tests, Li says, it was “sort of shocking to see that, if you just change the electrolyte, then all these cracks are gone.” They found that the morphology of the electrolyte material is much more robust, and the transition metals “just don’t have as much solubility” in these new electrolytes.
Their electrolytes' effectiveness at inhibiting cracking while permitting the battery's primary electrochemical processes to work was a happy surprise, he says. Upon closer examination, they learned that the new material still readily allows lithium ions to pass through—the essential mechanism by which batteries get charged and discharged—while blocking the other cations, known as transition metals, from entering. In addition, the accumulation of unwanted compounds on the electrode surface after many charging-discharging cycles was reduced more than tenfold compared to the standard electrolyte.
“The electrolyte is chemically resistant against the oxidation of high-energy nickel-rich materials, which prevents particle fracture and stabilizes the positive electrode during cycling,” says Shao-Horn, a professor of mechanical engineering and materials science and engineering. “The electrolyte also enables stable and reversible stripping and plating of lithium metal, an important step toward enabling rechargeable lithium-metal batteries with energy two times that of the state-of-the-art lithium-ion batteries. This finding will catalyze further electrolyte search and designs of liquid electrolytes for lithium-metal batteries rivaling those with solid-state electrolytes.”
Results So Far
The initial results are very promising, although additional work is required before the new electrolyte chemistry is ready to commercialize. Some of this will involve fine-tuning the chemistry and manufacturing process to sustain many charge-discharge cycles. One of the important factors in determining the cell's service life is managing the morphology of how the lithium is deposited during charging, i.e., developing mechanisms to ensure an even formation of the active layer and inhibit unwanted porosity due to trapped gases and liquids.
The researchers are already producing impressive results. They report that, in laboratory tests where lithium was alternately stripped and plated onto a copper substrate, the team's the LiFSI/DMTMSA electrolyte demonstrated an average coulombic efficiency of ~99% over 345 cycles. In addition, when the same chemistry was used in a prototype 4.7-V lithium-metal coin-cell battery, it delivered a specific capacity >230 mA h g−1 and an average coulombic efficiency of >99.65% over 100 cycles under normal use. It also retained >88% of its capacity after 90 cycles under harsh test conditions.
The next step is to develop techniques for scaling up production of the new electrolyte to volumes that make it affordable. “We make it in one very easy reaction from readily available commercial starting materials,” says Johnson. Right now, the precursor compound used to synthesize the electrolyte is expensive, but he says, “I think if we can show the world that this is a great electrolyte for consumer electronics, the motivation to further scale up will help to drive the price down.”
Because this is essentially a “drop in” replacement for an existing electrolyte and doesn’t require redesign of the entire battery system, Li says it could be implemented quickly and could be commercialized within a couple of years. “There’s no expensive elements; it’s just carbon and fluorine. So, it’s not limited by resources. It’s just the process,” he concluded.
Additional reading: "New electrode design may lead to more powerful batteries."
Reference
1. Xue, Huang, Li, et al, “Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte,” Nature Energy.