Pre-Charging vs. Formation in Lithium-Ion Cells
What you'll learn:
- How to stop the corrosion process after a cell is filled with electrolyte.
- Requirements for formation and pre-charging equipment.
- Why checking for reverse voltage is so critical during the manufacturing process.
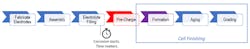
Manufacturing a lithium-ion cell is a complex, multi-step task. Whether you’re building a cylindrical, prismatic, or pouch cell, the fundamental steps are the same (Fig. 1). The formation step is key to the cell finishing process. During formation, the all-critical solid-electrolyte-interphase (SEI) layer is formed by passing current through the cell or charging the cell for the first time. Once the SEI is formed, you have a working cell that can be charged, retain energy, and discharged.
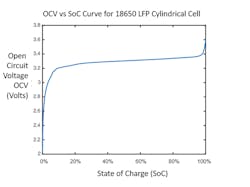
A working cell will have a maximum open circuit voltage (OCV) when the cell is fully charged, which is specified as 100% state of charge (SoC). This means that 100% of the energy the cell can store is held in the cell. It’s equivalent to saying your gas tank is 100% full.
As the cell is discharged, energy is removed from the cell, the SoC is reduced, and the OCV drops off (Fig. 2). Eventually, you will fully discharge the cell and reduce the SoC to 0%, where the cell’s OCV will be at a threshold called end-of-discharge voltage. As shown in Figure 2, this is 2 V; therefore, the operational range of the cell will be 100% SoC at 3.6 V down to 0% SoC at 2.0 V.
Process Steps: Filling, Pre-Charging, Forming
Going back to the manufacturing process, after electrolyte filling, the cell has no energy stored inside because no current has passed through the cell. What is the voltage on the cell at that moment? The exact answer depends on many factors, but the primary determinant is the materials used to construct the cell. Typically, you will see voltages immediately after electrolyte filling to be very low, ranging from 50 to 100 mV, but even small negative voltages down to −100 to −200 mV are possible.
Once the cell is filled with electrolyte, manufacturing process times become important. The electrolyte is an organic liquid, containing chemical additives to give the desired properties of the cell with respect to performance and safety. The liquid electrolyte will start to react with the materials on the surface of the electrodes. Corrosion may take place.
To stop the corrosion process from proceeding, the cell is pre-charged during the pre-charge step in the cell manufacturing process. Pre-charging takes that cell, with a very low voltage (typically from −100 to 100 mV), to about 2 V. This is both 0% SoC and a safe, stable voltage to avoid corrosion. Now, cells can move on to formation without concern about delays that could cause additional corrosion.
Equipment Requirements for Each Step
In formation, the cells will be charged with a low current for hours. The specific current used will depend on the size of the cell and on the specific protocol that the process engineers have selected to optimize SEI formation versus formation time. Using higher currents to speed up formation can negatively impact cell quality. Low currents are better for quality, but the increased formation time means more equipment (capital expenses) and formation time (operational expenses).
Most cell manufacturers hold such formation process information (i.e., what current and for how long) as company confidential information. In keeping with minimizing capital expenses, the formation equipment needs to be optimized for the job, as any extra capabilities will mean extra capital costs.
Thanks to the pre-charge step, the cells will enter the formation process at 2 V. This means formation equipment can be designed to operate over a very limited range from 2 V to the maximum OCV at 100% SoC of 3.6 to 4.2 V—depending on the cell chemistry. This minimum 2-V operating voltage on formation equipment contrasts with a typical cell test system or power supply/charger that will operate down to 0 V. The wider operating range on cell test equipment means the equipment will cost more. Thus, for formation, narrower-range, lower-cost, more specialized formation equipment is desirable.
But for pre-charge, the charging equipment requirements are different. During pre-charge, the cell is taken from a very low voltage, possibly even a negative voltage, and quickly brought up to 2 V by adding a brief charge to the cells. Low-voltage operation is required for pre-charge in contrast to formation, where low-voltage operation is undesirable. In fact, trying to apply standard formation equipment to pre-charging may not work, because it may not tolerate the pre-charge operating range with small negative voltages.
Safety
Let’s look at safety during pre-charge and formation. Preventing thermal runaway is the ultimate objective of safety protocols during cell manufacturing. Safety is a multi-faceted topic that I have in covered in part in a previous article. Therefore, I will focus on just one safety check that’s problematic for pre-charge testing: reverse cell detection.
If a cell is connected in reverse orientation, meaning equipment (+) to cell (–) and equipment (–) to cell (+), catastrophic results can happen if high current or significant energy is applied to the cell. As such, before applying energy to the cell, the cell is checked for reverse voltage at each electrical step of the process.
The method to detect reverse voltage is to measure the OCV of the cell and compare it to a threshold. For formation, checking for any cell voltage below 2 V can detect both a reversed cell and a faulty cell with an OCV that’s out of tolerance for that step of the process.
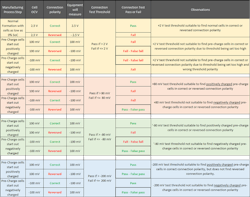
However, using this same approach to look for a reversed cell during pre-charge is ineffective, since the cell can have a very low positive voltage, zero voltage, and even a small negative voltage. The table highlights the issue.
Therefore, a voltage threshold test may not be the right answer. Instead, you can start the pre-charge without a reverse voltage check. Then, apply a small amount of energy, but not enough to initiate thermal runaway, and check the OCV again. If the OCV changed in the correct direction (i.e., became more positive), then the cell is oriented correctly. Alternatively, you can consider using a visual inspection (by an operator or machine vision) to observe if the cell is in the right orientation.
Summary
Remember these key points when weighing formation versus pre-charge:
- Formation equipment can be limited to operating at 2 V and higher to save on capital equipment expense, which is important in large-scale manufacturing where many formation channels are deployed due to long formation times.
- Pre-charge equipment needs to operate from a small negative voltage to 2 V, meaning standard formation equipment may not be designed to perform the pre-charge step.
- For pre-charge equipment, while operating in the small negative to 2-V range may increase the equipment cost, fewer channels are required because the pre-charge step is much shorter than the formation step.
- Detecting reversed cells during pre-charge is challenging, because the standard method of reverse cell detection can’t differentiate between a positively charged cell in the reverse orientation and a negatively charged cell in the correct orientation.
- Reverse cell detection alternatives include careful pre-charging and optical inspection.