3D-Printed Supercapacitor Fully—and Rapidly—Biodegrades
What you'll learn:
- How tailored chemical solutions provide the supercapacitor layers.
- How these solutions were 3D-printed onto a substrate, then folded to create the supercapacitor.
- How the final supercapacitor performs in use, and physically degrades when no longer needed.
Despite their specific differences, the Internet of Things (IoT), device tagging, and other monitoring/tracking applications all have one thing in common: They all need a power source, even if only a small amount and for a limited, definable timespan.
The options for providing such power include electrochemical batteries, supercapacitors, or energy harvesting (separately or in some combination). However, they all share one trait: When they’re no longer needed or viable—and many of these installations are shorter-term—they leave behind a power source that must be disposed of properly, or more likely, will become litter and waste.
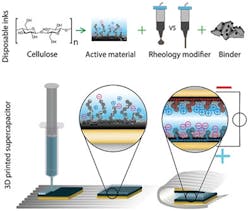
Now, a research team at EMPA (Swiss Federal Laboratories for Materials Science and Technology) has built print-on-demand supercapacitors using a modified, commercially available 3D printer along with their “not-so-secret sauce”—a recipe for the gelatinous inks that the printer can dispense onto a substrate as surface.
The mixture consists of cellulose nanofibers and cellulose nanocrystallites, plus carbon in the form of carbon black, graphite, and activated carbon. The researchers used glycerin, water, and two different types of alcohol plus a pinch of table salt (not for taste, of course, but for ionic conductivity) to properly liquify the solution and achieve the desired viscosity.
Despite the fragile sound of the design, this non-toxic capacitor isn’t a delicate, limited-use component. It can withstand thousands of charge/discharge cycles and years of storage, even in freezing temperatures, and is resistant to pressure and shock. At the same time, when it’s no longer needed, it can be placed in a compost pile or simply left “in nature.” After just a few months, it will have decomposed into harmless particles.
Developing the formulation and printing process was not a haphazard situation. "It sounds quite simple, but it wasn't at all," said Xavier Aeby of EMPA's Cellulose & Wood Materials lab. “It took an extended series of tests until all the parameters were right, until all the components flowed reliably from the printer and the capacitor worked.” He added, "As researchers, we don't want to just fiddle about, we also want to understand what's happening inside our materials."
Multilayered—But Not a PCB
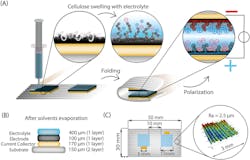
The supercapacitor needs four layers, which are produced by the 3D printer in sequence: a flexible substrate, a conductive layer, the electrode, and finally the electrolyte (Fig. 1). The researchers used a substrate made from ink composed of cellulose nanofibrils (CNFs), cellulose nanocrystals (CNCs), and glycerol as the base layer. The ink for the current collector contained graphite, carbon-black, and shellac. The electrode ink incorporated CNFs, CNCs, glycerol, activated carbon, and graphite particles. The ink for the electrolyte contained CNCs, glycerol, and NaCl.
These materials were printed on top of each other onto the substrate. They used direct-ink-writing (DIW), a printing technique in which a gel ink is extruded line-by-line and layer-by-layer to form 3D objects. The whole multilayer assembly is then folded up like a sandwich, with the electrolyte in the center (Fig. 2).
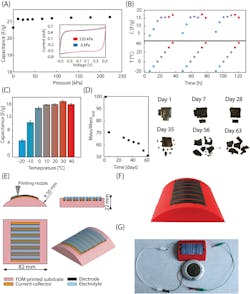
The resulting devices supported a high capacitance of 25.6 farads/gram of active material with an operating voltage up to 1.2 V. One was used to demonstrate a small clock (Fig. 3).
The team also tested performance over time and temperature. To clearly demonstrate the biodegradability of the construction, they also performed decomposition tests and found that after just two months, the capacitor disintegrated, leaving only a few visible carbon particles (Fig. 4).
The work is detailed in their paper “Fully 3D Printed and Disposable Paper Supercapacitors” published in Advanced Materials; it’s behind a paywall and there’s no open-access copy posted (but you can read the original for a modest up-front fee). There’s also open-access Supporting Information that has more details on the chemical composition of the inks, the setup parameters of the 3D printer, and additional test results. Also, check out this quick video: