“Simple” Optical Method Tracks Internal Battery Transitions During Charge/Discharge Cycles
What you’ll learn:
- How optical interferometric scattering microscopy can be used to look inside batteries during charge/discharge cycles.
- How internal observations reveal some of the constituent phase transitions within the battery.
- How the dynamics of charge/discharge cycles reveal battery characteristics.
The ongoing quest to view in fine detail what’s going on inside a battery as it’s charged and discharged continues, since such insight should, in principle, lead to improvements in battery cycling, life, safety, and overall performance. Among the techniques devised by researchers thus far involve complex arrangements using X-ray tomography, synchrotrons, and Raman-scattering microscopy (see References).
Now, researchers from the University of Cambridge (UK) have developed what they maintain is a superior operando (live viewing of an ongoing process) technique that’s much easier and quicker to implement, and which yields extreme details of “live” charging and discharging. Their approach resolves the fast/slow dilemma of these battery observations.
“To really study what’s happening inside a battery, you essentially have to get the microscope to do two things at once—it needs to observe batteries charging and discharging over a period of several hours, but at the same time it needs to capture very fast processes happening inside the battery,” said first author Alice Merryweather, a PhD student at Cambridge’s Cavendish Laboratory.
Exploiting iSCAT
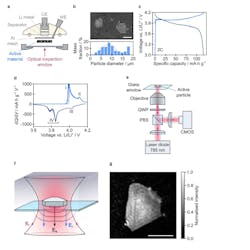
The team used optical interferometric scattering microscopy (iSCAT), which can detect and image a subwavelength object by causing interference with a reference light field and capturing the light scattered by the object (Fig. 1).
1. Electrochemical performance and interferometric scattering microscopy of a LCO electrode: (a) Geometry of the optical microscopy half-cell (WE = working electrode, CE = counter electrode). The counter electrode was lithium metal, the separator was glass fiber, and the cell stack was wet with standard carbonate liquid electrolyte (LP30). (b) Top: Scanning electron microscopy image of a dilute working electrode, showing two particles of LCO dispersed in a conductive matrix. Scale bar is 10 μm. Bottom: Mass-weighted diameter distribution for LCO particles (based on 681 particles). (c) Galvanostatic cycling (2C, 5 cycles) of the LCO electrode during operando optical measurements. (d) Corresponding differential capacity plots. The peaks attributed to biphasic transitions (I and IV) and lithium ordering (II and III) are indicated. (e) Optical setup of the interferometric scattering (iSCAT) microscope (PBS = polarizing beam splitter, QWP = quarter-wave plate, CMOS = complementary metal-oxide semiconductor camera). (f) Schematic diagram of iSCAT signal generation. Incident light (Ei) is focused onto an active particle of interest in the working electrode. The collected light includes a contribution scattered from the surface of the active particle (Es) and a reference contribution reflected from the top interface of the glass window (Er). (g) iSCAT image of a single active LCO particle in the electrode (250-μs exposure time). Intensity values are normalized to a range of 1. Scale bar is 5 μm.
A key feature of iSCAT is the detection of elastic scattering from subwavelength particles (an optical phenomena also known as Rayleigh scattering) in addition to reflected or transmission signals from supra-wavelength objects. Typically, the challenge is the detection of tiny signals on top of large and complex, speckle-like backgrounds (a scenario that’s analogous to many electronic signal-detection and -recovery challenges).
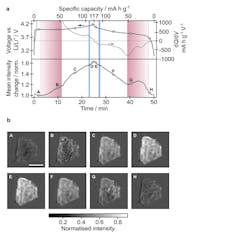
Using this technique, they were able to observe individual particles of lithium cobalt oxide (LixCoO2, often referred to as LCO) charging and discharging by measuring the amount of scattered light (Fig. 2). They were able to see the LCO going through a series of phase transitions in the charge-discharge cycle. The phase boundaries within the LCO particles move and change as lithium ions go in and out. The researchers found that the mechanism of the moving boundary is different depending on whether the battery is charging or discharging.
2. Overview of the optical response of an active particle during battery operation: (a) Top: Galvanostatic and differential capacity plots shown in black and grey, respectively, as a function of time (cycle 4, as plotted in Figure 1c and d). Bottom: iSCAT intensity change averaged over the active particle shown in Figure 1g, during galvanostatic cycling. Vertical red and blue shaded regions correspond to the durations of the biphasic and lithium ordering transitions, respectively, identified from images of this particle. (b) Background-subtracted iSCAT images of the active particle at the time-points indicated in panel a. Background subtraction was achieved by subtracting reference values for each pixel at the beginning of the cycle from the corresponding pixels in all subsequent images. Scale bar is 5 μm.
They determined the rates of lithium insertion and removal at the single-particle level and identified different mechanisms that occur on charge versus discharge. They also captured the dynamic formation of domain boundaries between different crystal orientations associated with the monoclinic lattice distortion at around Li0.5CoO2. The high-throughput nature of their technique allowed many particles to be sampled across the entire electrode and should, in the future, also enable exploration of the role of dislocations, morphologies, and cycling rate on battery degradation.
Charging Claims
Perhaps to get more attention, the University’s press release on their work makes some dramatic allusions as to its potential importance. For example, it states that it “could enable the batteries in most smartphones and laptops to charge in as little as five minutes.” Clearly, charge time also is a function of power-source current capacity, cabling, connectors, thermal considerations, and many other factors.
Nonetheless, improvements in a battery’s ability to accept charge is important to the overall situation. (They also tend to confuse a battery as an energy-storage device with a true energy source—that electricity for charging the battery has to come from somewhere).
Their paper “Operando optical tracking of single-particle ion dynamics in batteries” is published in Nature and focuses primarily on the test observations, results, and conclusions rather than the physical setup and instrumentation. While that paper is behind a paywall, an open version also is hosted at ArXiv (an open-access repository of electronic preprints and postprints approved for posting after moderation but not peer review, hosted by Cornell University). The paywall version does have open links at the end to a Supplementary Information posting as well as 10 short videos of various experiments in progress.
References
Solid-State Batteries Get Ready for Their (X-Ray) Closeup
Stimulated Raman Microscopy Provides Battery Ion-Transport Insight