EMI’s Potentially Dangerous Impact on Pacemakers
This article is part of the TechXchange: Delving into EMI, EMC and Noise
Members can download this article in PDF format.
What you'll learn:
- Medical advice for those with equipped with pacemakers and ICDs.
- How magnetic fields cause EMI problems.
- Devices that could wreak EMI havoc on pacemakers and ICDs.
Implantation of cardiac pacemakers and implantable cardioverter defibrillators (ICDs) are on the rise. And the population of working-age people with ICDs also is increasing. Medical experts are experiencing growing concerns regarding electromagnetic interference (EMI) that may seriously affect these implanted devices, especially in a work environment with high-level electromagnetic-field (EMF) levels.
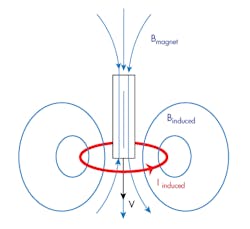
Many factors that can affect EMI are difficult to foresee; however, the reduction in field strength with distance is usually predictable. Faraday’s Law states that the induced voltage is proportional to the induction area (Fig. 1).
There are two main variables for EMI:
- Distance of the device from the EMI source
- Duration of exposure
The intensity of an electric or magnetic field will decrease with the square of the distance. If a pacemaker or ICD patient doubles their distance from the source, he or she will be exposed to only one quarter of the original field.
Pacemaker and ICD Patients Must Be Aware of Medical Advice
The latest medical advice suggests:
- After a patient receives a pacemaker or ICD, a risk assessment regarding EMI needs to be done to determine if the patient can return to work.
- ICDs and the latest pacemakers, having bipolar sensing configurations, are equipped with adequate shielding against low-frequency magnetic fields.
- Pacemakers programmed to unipolar sensing may be susceptible to EMI in a working environment.
EMI in the 10- to 60-Hz Frequency Range
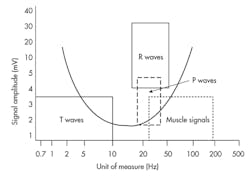
EMI signals in the 10- to 60-Hz frequency range can negatively affect cardiac devices since they overlap the cardiac signal range. This is because amplitude and frequency of human muscle potentials overlap the same range as cardiac signals.
Oversensing of myopotential signals is common in unipolar sensing systems. Myopotential signals commonly reach the 2- to 4-mV amplitude range. Bandpass filters allow for only selected frequencies to pass through the sensing circuit. Typical ranges for P-waves are 20 to 40 Hz, R-waves 18 to 50 Hz, and T-waves 0 to 10 Hz (Fig. 2).
Ambient Magnetic Fields
Static magnetic fields (0 Hz) produced by devices with a permanent magnet have proven to result in EMI in pacemakers/ICDs and their interrogation telemetry. This kind of exposure can impact a pacemaker switch, causing it to switch into an asynchronous pacing mode of operation.
Common devices that may produce a magnetic field include computer hard drives, portable media drives, wireless headphones, and small neodymium magnets. Other applications with a magnetic field are attachments in corrective devices or for holding together replacement dentures where several teeth are missing. Audio microphones, loudspeakers, jewelry, and bearings also can generate magnetic fields.
Tests regarding neodymium magnets were performed and magnetic interference was observed in all patients.2 The maximum distance resulting in device interference was 3 cm. No significant differences were found with respect to device manufacturer and device types.
Small neodymium-iron-boron (NdFeB) magnets were shown to cause interference with cardiac pacemakers and ICDs. Patients should be cautioned about the interference risk associated with NdFeB magnets during daily life. Magnetic decorations on clothing can be beautiful,3 but can also be a potential harm to pacemaker operations.
Tests were also done using welding equipment.1 The results revealed that there was interference with bradycardia (slow heartbeat patients) with pacemakers set in unipolar sensing mode, and no interference from EMF exposure in bradycardia (slow heartbeat patients) with pacemakers set in bipolar mode.
Other Devices that May Interfere with ICDs and Pacemakers
Many devices and machinery may interfere with ICDs and pacemakers. Electromagnetic waves need to be avoided or at least minimize the exposure to them. Examples of devices/systems to avoid include:
- Anti-theft systems such as those found in department stores are usually okay and will not cause significant symptoms in the majority of patients. However, it’s best to minimize being close to these devices.
- Security metal detectors are usually unlikely to affect ICDs or pacemakers; however, don’t stay near these devices too long and don’t lean on them. If patients need to be scanned by handheld metal detectors, they should advise security personnel that they have an ICD or pacemaker. Patients can request alternate forms of inspection like a pat down.
- Cell-phone antennas are low risk to ICDs and even less risk for pacemakers. Newer cell phones with the latest frequency capabilities can possibly make ICDs and pacemakers less reliable. It might be best to keep the cell phone a minimum of six inches away from an ICD or pacemaker. Also, users should not keep a cell phone in a front chest pocket.
- Walkie-talkies at 3 W power or less should be kept a minimum of six inches from the ICD or pacemaker device.
- The good news is that Bluetooth headsets seem to not interfere with ICDs or pacemakers.
- Be aware that headphones have magnetic materials which may interfere with ICDs and pacemakers (earbuds are okay). Keep these at least six inches away from ICDs or pacemakers. Don’t put these devices in your breast pocket or hang them around your neck, draped on the chest.
- CB or ham radios under 3 W should be kept a minimum of six inches from ICDs; 3- to 15-W power radios should be 12 inches minimum distance and 15 to 30 W or more should be at least two feet away from ICDs. Pacemakers have little or no risk here.
- Beware of power-generating equipment, arc welding devices, and jumper cables. Best to stay at least two feet away.
- Magnetic-resonance-imaging (MRI) equipment have powerful magnets. In general, stay away from these. Follow your doctor’s advice for such imaging and ask if maybe there’s an alternative form of imaging equipment. Always inform a technician that you have an implantable device.
Test and Evaluation of Potential Cell-Phone Interference
The FDA has taken a leading role in developing detailed test methodologies to measure EMI from cell phones in implanted cardiac pacemakers and defibrillators. Their test method is now part of an international standard (ISO 14117) and is used for testing and evaluation of potential EMI from cell phones.
The FDA continues to monitor the use of cell phones for possible interactions with other electronic medical devices. This organization will develop new assessment methods to help ensure minimal or no impact on the function of these devices due to EMI.
Summary
Significant advances have been made in electronic technology for pacemakers/ICDs. Implementing hermetic shielding, filtering, bipolar sensing, and algorithms, all designed to reject sources of EMI, have really helped return patients with pacemakers back to active lives in their communities after pacemaker/ICD implantation.
However, new technologies have increased concern about interference with the pacemaker/ICD function. It’s important for doctors and medical technicians to keep updated regarding the potential risks of EMI from modern external sources with respect to proper pacemaker/ICD function.
Read more articles in the TechXchange: Delving into EMI, EMC and Noise
References
1. Electromagnetic interference with cardiac pacemakers and implantable cardioverter-defibrillators from low-frequency electromagnetic fields in vivo, European Society of Cardiology.
2. Potential interference of small neodymium magnets with cardiac pacemakers and implantable cardioverter-defibrillators, National Library of Medicine.
3. Magnet decoration, beautiful but potentially dangerous for patients with implantable pacemakers or defibrillators, National Library of Medicine.
4. Pacemakers for bradycardia, Medtronic.
5. Electromagnetic Interference of Pacemakers, Intechopen.com.
6. Devices that May Interfere with ICDs and Pacemakers.
7. Electromagnetic Interference: Guide to electromagnetic compatibility and your cardiac device, Abbott.
8. Electromagnetic compatibility guide for implantable cardiac devices, Medtronic.