Battery Dendrites Shown to Form from Mechanical Stress
What you’ll learn
- Battery dendrites are not caused by electrical origins, but instead have mechanical sources.
- The dendrites propagate due to fracture of the electrolyte and subsequent crack filling-in.
- Stresses on the order of 200 MPa can deflect and arrest dendrites in oxide electrolytes.
Dendrites are projections of metal that can build up on the lithium surface within a battery and penetrate into the solid electrolyte (their name comes from the Latin for branches). They eventually cross from one electrode to the other and short out the battery cell.
Their existence and ongoing growth has been one of the impediments to reducing the overall size and weight of the battery and minimizing the safety risk associated with the potentially flammable liquid electrolytes. This improvement be achieved by replacing the liquid electrolyte with a much thinner, lighter layer of solid ceramic material, and replacing one of the electrodes with solid lithium metal.
Due to their highly undesirable impact, researchers have been studying dendrites extensively for many years to better understand their formation and growth. However, progress has been slow, with conflicting theories as to what initiates or encourages them, or how to prevent them.
Even the question of whether dendrites are driven by mechanical failure or electrochemical degradation of solid electrolytes remains somewhat unanswered. If internal mechanical forces drive failure, superimposing an external compressive load that counters internal stress may mitigate dendrite penetration.
Now a joint research team from two departments at MIT (one being their delightfully named “Center for Bits and Atoms“) and Brown University appears to have resolved the issue of dendrite formation and how to prevent them from crossing through the electrolyte. They did this by dynamically applying mechanical loads to growing lithium metal dendrites in Li6.7 La3Zr1.75Ta0.25O12 solid electrolytes.
Live operando microscopy revealed marked deflection in the dendrite growth trajectory at the onset of compressive loading. At loads near 200 MPa (about 20,000 psi), this deflection was sufficient to avert cell failure (Fig. 1).
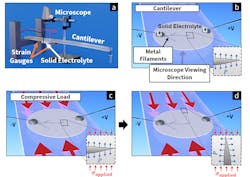
1. Observing the response of metal dendrites to applied loads:
(a) A microscope observes a solid-state cell consisting of lithium metal electrodes adhered to a thin solid electrolyte plate (1/2 in. diameter), fixed rigidly to a transparent cantilever. Weight applied to the end of the cantilever induces strain in the bar and the electrolyte. This strain is measured in real-time using strain gauges.
(b) The plan-view cell geometry and dendrite orientation in the load-free configuration. Applied current produces plating-induced pressure (𝑃𝑃) inside metal-filled flaws at the anode/electrolyte interface. This pressure acts normally to the flaw surface, wedging open the flaw and allowing metal dendrites to propagate through the cell.
(c) The cell under load. Weight placed on the end of the cantilever (see “a”) generates compressive strains in the cantilever and the electrolyte. Resulting compressive stress (𝜎applied) acts along the cantilever’s axis and opposes the plating-induced pressure 𝑃, causing crack opening and dendrite propagation.
(d) Dendrite deflection when propagating under compressive load. For 𝜎applied = 200 MPa, the metal propagation direction turned about 90 degrees to align with the loading axis.
It’s All Mechanical
Previously, some researchers thought that dendrites were formed by a purely electrochemical process rather than a mechanical one. The team’s experiments demonstrate that it’s mechanical stresses causing the problem. Using fracture mechanics and associated in-depth analysis, they quantified the impact of stack pressure and in-plane stresses on dendrite trajectory, charted the residual stresses required to prevent short-circuit failure, and even proposed cell-design approaches to achieve such stresses.
Not surprisingly, the setup was complicated. Dendrite formation normally takes place deep within the battery cell and can’t be observed directly. So, they developed a way of making thin cells using a transparent electrolyte, thus allowing the whole process to be directly seen and recorded. The team demonstrated that they could directly manipulate the growth of dendrites simply by applying and releasing pressure, causing the dendrites to zig and zag in perfect alignment with the direction of the force.
At first, they assumed that a stress direction called stack pressure (which is often applied to battery cells by “squishing” the material in the direction perpendicular to the battery’s plates, like compressing a sandwich by putting a weight on top of it) might help prevent the layers from separating. But their experiments have demonstrated that pressure in that direction actually exacerbates dendrite formation accelerates dendrite-induced failure.
Preventive Measures
Instead, what’s needed is pressure along the plane of the plates (to continue the analogy, it’s as if a sandwich was being squeezed from the sides) to force the dendrites to travel in the direction of the compression.
Applying mechanical stresses to the solid electrolyte doesn’t eliminate the formation of dendrites, but it does control the direction of their growth. This means they can be directed to remain parallel to the two electrodes and prevented from ever crossing to the other side, thus rendering them harmless.
The shuttling back and forth of ions causes the volume of the electrodes to change. That inevitably causes stresses in the solid electrolyte, which must remain fully in contact with both of the electrodes between which it is sandwiched.
“What we have shown in this work is that when you apply a compressive force you can force the dendrites to travel in the direction of the compression,” noted graduate student Cole Fincher, and if that direction is along the plane of the plates, the dendrites “will never get to the other side.”
“To deposit this metal, there has to be an expansion of the volume because you’re adding new mass,” said MIT Professor Yet-Ming Chiang. “So, there’s an increase in volume on the side of the cell where the lithium is being deposited. And if there are even microscopic flaws present, this will generate a pressure on those flaws that can cause cracking.”
Those stresses, the team has now shown, cause the cracks that allow dendrites to form. The solution to the problem turns out to be more stress, applied in just the right direction and with the right amount of force.
Further, the team demonstrated that they could directly manipulate the growth of dendrites simply by applying and releasing pressure, causing the dendrites to zig and zag in perfect alignment with the direction of the force (Fig. 2).
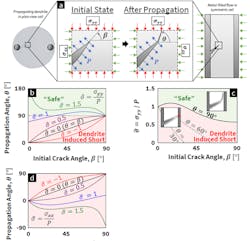
2. Predicting dendrite deflection based on mixed-mode fracture mechanics:
(a) Schematic of loading conditions used in modeling kinked propagation of metal dendrites. The most energetically favorable propagation angle 𝜃 as a function of the flaw inclination 𝛽 is obtained. Both angles are measured counterclockwise from the horizontal. Two loads are present for this flaw: a plating-induced stress P acting normal to the flaw surface, with an additional load (𝜎𝑥𝑥 and/or 𝜎𝑦𝑦) due to external forces or from residual stresses present in the solid electrolyte. This model applies to two (equivalent) loading scenarios: 1) The kinking of propagating metal dendrites upon the application of applied load, shown for a plan-view cell on the left side of the subfigure, and 2) the kinking of a metal-filled flaw at the anode/electrolyte interface at the instant propagation begins (seen in the right side of the subfigure).
(b) The most energetically favorable propagation angle as a function of initial crack-inclination angle 𝛽 for different values of 𝜎 = 𝜎𝑦𝑦/𝑃. The black curve represents the case where the only stress in the system is 𝑃, (i.e., 𝜎 = 0), such that 𝜃𝜃 = 𝛽. Increasingly positive 𝜎 values represent increasing compressive loadings, which then increase the value of 𝜃 relative to that for 𝜎 = 0, causing deflection. In the limit where 𝜃 = 90°, the metal dendrite can’t reach the counter electrode regardless of the lateral dimensions of the electrolyte.
(c) The value of 𝜎 required to produce 𝜃 = 90°, 𝜃 = 60°, and 𝜃 = 30°as a function of crack inclination 𝛽.
(d) The most energetically favorable propagation angle 𝜃 as a function of inclination angle 𝛽 for different values of 𝜎 = 𝜎𝑥𝑥/𝑃. Increasingly positive values of 𝜎 represent increasing compressive stack pressures, where stack pressures approaching the magnitude of 𝑃 tend to decrease the propagation angle relative to 𝜎 = 0.
In their tests, the researchers used pressure induced by bending the material, which was formed into a beam with a weight at one end. But they say that in practice, there could be many different ways to produce the needed stress.
In summary, their conceptual model and subsequent experiments show that in the materials studied, dendrite propagation is dictated by fracture of the electrolyte and that electronic conductivity plays a negligible role. The work is detailed—and I do mean detailed—in their paper “Controlling dendrite propagation in solid-state batteries with engineered stress” with its extensive analytical section published in Joule. While that paper is behind a paywall, an open-access copy is posted at chemrxiv.org.