Lithium-Free Battery Aims to Serve as Backup for Grid-Scale Renewable Energy
What you'll learn:
- Details behind PNNL's molten-salt battery design.
- Storage capabilities possible as displayed by the prototype.
- Can the battery meet grid-scale storage requirements?
A research team, led by the Department of Energy’s Pacific Northwest National Laboratory (PNNL), demonstrated a new design for a grid energy-storage battery that uses sodium and aluminum as its primary components. A small prototype cell has validated the cell's novel molten salt chemistry's ability to perform well, retain capacity through hundreds of charge/discharge cycles, and operate at much lower temperatures than earlier designs.
The cell is well-suited for long discharge cycles, in excess of 24 hours. Its architecture will enable the construction of flat, scalable batteries that can more easily be stacked for industrial- and grid-scale applications.
According to a study just published in Energy Storage Materials, the new design, built with abundant, inexpensive metals, could help ease integration of renewable energy into the nation’s electrical grid at lower cost.
“We showed that this new molten-salt battery design has the potential to charge and discharge much faster than other conventional high-temperature sodium batteries, operate at a lower temperature, and maintain an excellent energy storage capacity,” said Guosheng Li, a materials scientist at PNNL and the principal investigator of the research. “We are getting similar performance with this new sodium-based chemistry at over 100°C [212°F] lower temperatures than commercially available high-temperature sodium battery technologies, while using a more Earth-abundant material.”
A Better Energy-Storage Solution
The new sodium-based molten-salt battery is based on two different electrochemical reactions that build upon the single neutral molten-salt reaction they had previously developed. The new discovery shows that this neutral molten salt can undergo an additional reaction that results in an acidic molten salt. Crucially, this second acidic reaction mechanism increases the battery’s capacity.
The recently demonstrated lab-scale "coin cell" prototype has a capacity of 416 mAh. PNNL researchers anticipate using what they learn to develop early-stage practical batteries with capacities of around 5000 mAh (5Ah). Although the battery is in initial development, the researchers speculate that the technology is likely to eventually support practical energy densities of up to 100 Wh/kg.
While not as high as the energy densities of lithium-ion batteries utilized in commercial electronics and electric vehicles (170 to 250 Wh/kg), the sodium-aluminum battery design is much less expensive and easier to produce from materials that are widely available in the in the United States.
Meeting Grid-Scale Storage's Unique Requirements
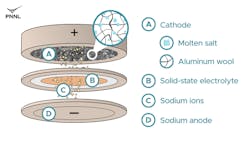
The new battery was assembled and tested as part of a collaboration between PNNL and the U.S.-based renewable-energy pioneer Nexceris. Their new business, Adena Power, supplied its patented solid-state, sodium-based electrolyte to PNNL to test the battery’s performance. This critical battery component enables the sodium ions to travel from the negative (anode) to the positive (cathode) side of the battery as it charges.
After the prototype cell was subjected to 345 charge/discharge cycles at high current, its acidic reaction mechanism retained 82.8% of its original peak charge capacity. While this is promising, it will take further development to be able to endure 1,000 cycles while retaining 90% of its original capacity (i.e. the minimum performance considered commercially viable utility-scale storage system).
Guosheng Li said that to meet this goal, they will work on optimizing the Al cathode and the battery cell's cycling conditions. He expects their efforts will greatly extend cycle life within three years.
More Capacity at a Lower Cost
As part of the study, the researchers estimated that the active materials required for a sodium-aluminum battery could cost as little as $7.02 per kWh. The researchers expect that this cost could be even further reduced as the design is optimized and new developments improve its practical energy density.
Li noted that the inexpensive material costs should enable the commercial production of large, long-duration batteries capable of supporting 10- and 24-hour discharge periods, with prices in the neighborhood of $70/kWh and $168/kWh, respectively.
“Our primary goal for this technology is to enable low-cost, daily shifting of solar energy into the electrical grid over a 10- to 24-hour period,” said Vince Sprenkle, a PNNL battery technology expert with more than 30 patented designs for energy-storage systems and associated technology. “The good reaction kinetics of the aluminum cathode and the high ionic conductivity of the NaAlCl4 molten salt provide the high areal loading capacity of the cathode, which is crucial for extending the discharge duration. This is a sweet spot where we can start to think about integrating higher levels of renewables into the electrical grid to provide true grid resiliency from renewable resources such as wind and solar power.”
Sprenkle was part of the team that developed this battery's new flexible design. The development also shifted the battery from a traditional tubular shape to a flat, scalable one that can more easily be stacked and expanded as the technology progresses from coin-sized batteries to a larger grid-scale demonstration size.
More importantly, this flat cell design allows the cell capacity to be increased by simply using a thicker cathode, which the researchers leveraged in this work to demonstrate a triple capacity cell with sustained discharge of 28.2 hours under laboratory conditions.
This contrasts sharply with most current battery technologies, including lithium-ion batteries, which can support much shorter discharge periods, typically in the range of four hours. That’s sufficient for buffering the short-term fluctuations in output experienced by wind and solar farms during normal operation. However, the ability to support discharge periods of 10-plus hours are needed to shift renewable sources' peak capacity to meet evening load demands, or when adverse weather takes them offline extended periods.
Bridging the Capacity Gap
PNNL has been developing technologies to meet the demand for grid-scale storage for nearly a decade, including a sodium-based freeze-thaw battery that's capable of storing energy generated seasonally for months at a time. While the freeze-thaw system excels at shifting energy on a seasonal timeframe, this new design is particularly suited for short- to medium-term grid energy storage over 12 to 24 hours. It’s a variation of what’s called a sodium-metal halide battery, which has been successfully used in a commercially available battery based on a more expensive nickel cathode.
“We have eliminated the need for nickel, a relatively scarce and expensive element, without sacrificing battery performance,” said Li. “Another advantage of using aluminum over nickel is that the aluminum cathode charges more quickly, which is crucial to enable the longer discharge duration demonstrated in this work.”
With this milestone reached, the team is focusing on further improvements to increase the discharge duration, which could greatly improve grid flexibility for greater incorporation of renewable power sources.
A Successful Collaboration
The research was supported by the DoE Office of Electricity and the International Collaborative Energy Technology R&D Program of the Korea Institute of Energy Technology Evaluation and Planning. The electrolyte development was supported by a DoE Small Business Innovation Research program. The nuclear magnetic resonance measurements were made in the Environmental Molecular Sciences Laboratory (EMSL), a DoE Office of Science User Facility sponsored by the Biological and Environmental Research program.
Learn more about PNNL’s grid modernization research and the Grid Storage Launchpad, opening in 2024.