What you'll learn:
- How researchers used X-ray microtomography imaging to look inside a battery cell during charge/discharge.
- Results obtained using the synchrotron-based technology.
Battery cells present an interesting dilemma when it comes to R&D, test, and evaluation. On one side, their most-important parameters of terminal voltage, current flow, and temperature are easily observed. On the other hand, their interior situation and workings are very hard to observe, especially in real-time. However, understanding what’s happening inside in detail is critical to improved battery performance and advances.
Now, a multi-institution team led by a group at Georgia Institute of Technology (Georgia Tech) including Argonne National Laboratory has advanced a technique for looking inside the battery cell while it’s being charged and discharged. This X-ray microtomography imaging is similar to a medical CAT scan (note: in operando refers to a scan while the test subject is “operating” and fully functioning rather than non-functioning in situ or “on site”).
The team was able to observe the internal “evolution” of the materials inside solid-state lithium batteries, which use solid materials to replace the flammable liquid electrolytes in existing lithium-ion batteries. They created detailed, three-dimensional information that could help improve the reliability and performance of the batteries.
The operando synchrotron X-ray “computed-microtomography” imaging revealed how the dynamic changes of electrode materials at lithium/solid-electrolyte interfaces determine the behavior of solid-state batteries. The researchers found that battery operation caused voids to form at the interface, creating a loss of contact that was the primary cause of failure in the cells.
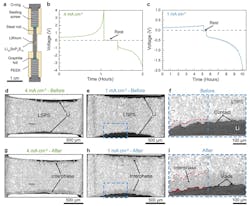
They noted that despite progress in solid-state battery engineering, any advanced understanding of the chemo-mechanical phenomena that controls electrochemical behavior and stability at solid-solid interfaces remains limited compared to solid-liquid interfaces. Using synchrotron-based X-ray computed microtomography, they investigated the changes in lithium/solid-state electrolyte interfaces during battery cycling in a test cell at relatively high current densities of 1 mA/cm2 and 4 mA/cm2 (Fig. 1).
1. A smaller, modified version of the cell shown here was used to image these materials during cycling.
The tests provided insight into the complex interplay between void formation, interphase growth, and volumetric changes, all of which determine cell behavior. They were able to directly and dynamically visualize the formation of voids during lithium stripping, and found that the resultant loss of contact at the interface between lithium and the solid-state electrolyte (Li10SnP2S12) was the primary cause of cell failure (Fig. 2).
2. Operando X-ray imaging of cells at two current densities. (a) Schematic of the custom X-ray tomography cell used to cycle Li/LSPS/Li cells during operando experiments. (b-c) Galvanostatic voltage curves measured during operando experiments at 4 mA/cm2 and 1 mA/cm2, respectively. (d-e) Reconstructed cross-sectional images before cycling at 4 mA/cm2 and 1 mA/cm2. The regions with dark contrast are the lithium electrodes, while the gray phase is the LSPS electrolyte. (f) Magnified cross-section of the Li/LSPS interface before cycling at 1 mA/cm2, taken from the blue-boxed region in (e). Voids in the left half of the image are overlaid with red for easier visualization, and the red dashed line on the left side demarcates the interphase boundary. The right half of the image is unmarked. (g-h) The same cross-sectional images as those shown in (d-e) after the electrochemical cycling procedure shown in (b-c). The formation of a darker gray interphase can be seen at the interfaces, along with morphological changes in the lithium electrodes. (i) Magnified cross-section of the same interface as shown in (f) after one full cycle at 1 mA/cm2. The volume of voids at the interface has increased significantly (overlaid with red on the left half of the image), along with growth of the interphase (demarcated by the red dashed line in the left half of the image).
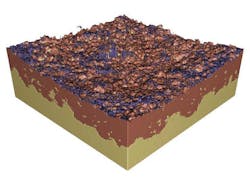
They were also able to construct a three-dimensional view of the lithium/solid-electrolyte interface within the battery (Fig. 3).
3. A three-dimensional view of the lithium/solid-electrolyte interface within the battery was reconstructed with X-ray tomography.
Team leader Matthew McDowell, an assistant professor in the George W. Woodruff School of Mechanical Engineering and the School of Materials Science and Engineering, remarked that “we were able to understand exactly how and where voids form at the interface, and then relate that to battery performance.”
Details of the project with numerous charts, data, process arrangement, and conclusions are in their Nature Materials paper “Linking void and interphase evolution to electrochemistry in solid-state batteries using operando X-ray tomography” along with Supplementary Information. The primary paper is behind a paywall, but it’s also available here. The research used resources of the Advanced Photon Source operated by Argonne National Laboratory.
Reference
NPG Asia Materials, “In situ/operando synchrotron-based X-ray techniques for lithium-ion battery research” (2018)