This file type includes high resolution graphics and schematics.
Advances in electronic hardware and software are allowing us to live longer. They’re driving improvements in biomedical diagnostics, treatments, and therapies with implantable organs and devices, more accurate and targeted drug delivery, and greater communications between healthcare providers and patients. Biomedical and electronics technologies are forming a permanent symbiotic relationship.
State-of-the-art artificial organs and prosthetics are becoming more widely available, many with federal Food and Drug Administration (FDA) approval, to replace just about any missing or failing organ or limb. Combined with impressive advances in wireless sensor networks, these developments are prolonging lives, improving the quality of those lives, and making medical healthcare more portable and interactive.
Related Articles
Most patient monitoring and therapy applications now involve disposable microelectromechanical systems (MEMS) sensors. These devices are used for flow sensing in ventilators and respirators, implantable devices, cardiac monitoring, and infusion pumps that deliver fluids, medications, or nutrients into the patient’s circulatory system.
Market analyst ABI Research estimates that by 2018, close to 5 million disposable biomedical sensors will be shipped. Many of these sensors will be used in body-area network applications, which are in their infancy and starting to grow.
Analog and digital semiconductor IC manufacturers are contributing heavily to the biomedical industry with filters, microphones, microcontrollers, DSPs, transceivers, amplifiers, FPGAs, systems-on-chip (SoCs), and memory chips. These ICs perform significant roles in nerve stimulation, drug delivery, and wireless communications in implantable electronics.
The challenge for these chips is to overcome their limitations. Their relatively large package sizes need to be smaller. They need to dissipate less and less power. They must show greater biocompatibility with the human body. And, they have to address critical reliability and safety issues.
Perfecting Implants
Organ implants are becoming better and more sophisticated. For example, Switzerland’s Debiotech and STMicroelectronics collaborated on the JewelPump, which delivers insulin using a MEMS microfluidic chip to treat diabetes. Implantable insulin pumps are becoming available to make such delivery more accurate as they closely resemble the way insulin is secreted normally. According to the World Health Organization, 347 million people worldwide suffer from diabetes.
The patient wears the sleek, 25-gram unit on the arm as a patch pump that holds 500 units of insulin, enough for seven days without the need to refill or replace it (Fig. 1). The pump is attachable to an auto-inserted canula patch that can be replaced every three days without having to change or refill the pump.
To make the JewelPump easier, safer, and more accurate, Debiotech developed the JewelCOM, a state-of-the-art PDA-phone. It works on an open Android operating system and includes a slot for the patient’s subscriber identity module (SIM) card for security as well as a highly precise glucostrip (JewelSTRIP) for accurate blood glucose dosages. Debiotech has collaborated with Switzerland’s Valtronic to develop an electronic controller unit for the JewelPump as well.
Debiotech is also supplying the Nanopump, a miniaturized pump for insulin delivery. It uses a volumetric membrane pump, with a pair of check-valves, integrated on a MEMS chip that consists of three layers bonded together: a silicon-on-insulator (SOI) plate with micromachined pump structures and two Pyrex cover plates with through-holes. A piezoelectric actuator moves the membrane in a reciprocating movement to compress and decompress the pump’s fluid in the pumping chamber. STMicroelectronics will manufacture the Nanopump.
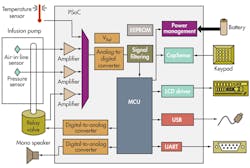
SoC ICs play a critical role in implantable insulin-pump drug delivery, which is tricky and complex since user control is needed to administer bolus and basal doses. A bolus dose is pumped to cover food eaten or to correct for a high blood glucose level. A basal dose is pumped continuously at an adjustable basal rate to deliver insulin between meals and at night. SoCs like the programmable SoC (PSoC) family from Cypress Semiconductor enable patients to modify the profile of the delivered insulin depending on the body’s need for it and the time of day and night it is administered (Fig. 2).
The FDA has approved Medtronic’s Viva portfolio of cardiac resynchronization therapy (CRT) with defibrillation capability (CRT-D) and Evera portfolio of implantable cardioverter defibrillators, or ICDs (Fig. 3). An adaptive algorithm, AdaptiveCRT, is used to improve heart-failure patients’ response rate by 12% compared with historical CRT trials, according to Medtronic.
A study from the University of Michigan’s Department of Aerospace Engineering has found that pacemakers can be powered by heartbeats themselves using the piezoelectric approach. “Today’s pacemakers must be replaced every five to seven years when their batteries run out, many of which are worn by children, which is costly and inconvenient. You can imagine how many operations they are spared if this new technology is implemented,” said M. Amin Karami, lead author of the study and a research fellow.
This file type includes high resolution graphics and schematics.
An Artificial Heart Ready For Trial
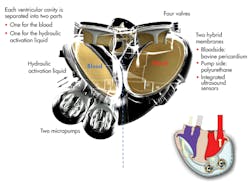
An artificial heart from France’s Carmat SAS will begin testing in human patients shortly in four renowned international cardiac surgery centers in Belgium, Poland, Saudi Arabia, and Slovenia. The prosthesis consists of two ventricular cavities comprising two volume spaces, each separated by a flexible bio-membrane, one for blood and one for the actioning fluid. The fluid is contained in an external bag that beats at the same rate that a native heart would (Fig. 4).
Each cavity is separated into two parts, one for the blood and one for the hydraulic activation liquid. Two micropumps power the implant. An electronic device regulates the operation of the prosthesis according to the needs of the patient, based on information provided by the sensors and processed by a microprocessor.
A wearable communication system provides information and relays diagnostic data to the hospital for remote monitoring. Lithium-ion batteries will power the first-generation hearts and allow patients to return home under good conditions with a portable system carried on a trolley or on their shoulder. Second-generation hearts will be powered by fuel cells worn directly by the patient. Carmat is waiting for final regulatory approval from France’s ANSM, the French National Security Agency for Drugs and Medical Products.
The University of Pittsburgh is developing a wearable artificial lung that would serve as a bridge to transplants or recovery in patients with acute and chronic lung failure. The Parcorporeal Ambulatory Assist Lung (PAAL) is being funded by a $3.4 million grant from the U.S. National Institute of Health (NIH). It’s a wearable fully integrated blood pump and lung designed to provide respiratory support for up to three months while maintaining excellent blood compatibility.
The Eyes And Ears Have It
A new retinal prosthesis is bringing people from the darkness into the light. The FDA has approved the Argus II retinal implant from Second Sight Medical Products Inc.,promising to partially restore sight to blind patients who suffer from retinitis pigmentosa.
The patient sees a low-resolution image with levels of gray much like a black and white TV. It’s all done with a video camera in a pair of glasses, a small computer, and an eye implant. The camera picks up images in front of patients. It then wirelessly transmits signals to an implant inside the eye and electrically stimulates the remaining tissue in the retina. Patients have reported perceiving spots of light that recreate images and movement.
Powering retinal implants via solar energy is the goal of researchers at Stanford University’s School of Medicine. Solar cells would be surgically placed underneath the retina to power a new kind of retinal prosthesis that involves a pair of goggles equipped with a miniature camera and a pocket PC that processes the visual data stream. The resulting images are displayed on a liquid-crystal micro-display embedded in the goggles, similar to what is used in video goggles for gaming. Experimental studies on rats have shown that photodiodes in the rats’ retinas produced accepted visual indicators of visual activity. The next step is to test the system on humans.
Cochlear implants for the hearing impaired are now a reality. Advanced Bionics offers the HiResolution bionic Ear System. The company has honed its ClearVoice software algorithm for implants using a combination of high-fidelity front-end AutoSoun signal processing and innovations in stimulus delivery (current steering and high simulation rates) that work with its HiRes Fidelity 120 using its Harmony and Neptune sound processors.
Even those hearing impaired patients who don’t need an implant can benefit from a low-noise omni-directional MEMS microphone for hearing aids. Compared to conventional electret microphones, the ADMP801 from Analog Devices is smaller at 7.3 mm3 and produces an equivalent input noise (EIN) of 27-dBa SPL (sound pressure level). It also consumes just 17 µA with a 1-V supply, which is fraction of what electret microphones consume.
Powering cochlear implants via energy harvesting is the goal of researchers at the Massachusetts Institute of Technology (MIT), the Harvard University-MIT Division of Health Sciences and Technology, and the Massachusetts Eye and Ear Infirmary. They have harvested the energy of a guinea pig’s inner ear to power a small sensing device.
The electrical potential of the ear’s cochlea operates like a biological batter, turning sound signals into electrical signals sent to the brain. The researchers developed a chip that can harvest this electrical energy without interfering with normal hearing. It enables the monitoring of biological activity in the ears of those with hearing or balance impairments as well as their responses to therapy. Eventually, the chip might even deliver the needed therapy by itself.
Prosthetics Are Improving
A small revolution has been brewing in the development and manufacture of artificial limbs. Faced with hundreds of armed forces service members sustaining amputations during the wars in Iraq and Afghanistan, advances in sensors, microprocessors, materials, and manufacturing techniques have allowed “prosthetic limbs to better adapt to patients, not the other way around,” said William Hanson, president of Liberating Technologies.
Among its many prosthetic products, Liberating Technologies offers the Boston Digital Arm System for above-elbow amputees (Fig. 5) and the VariGrip prosthetic controller for below-elbow amputees. These state-of-the-art microprocessor-based prosthetic controllers can be customized to accommodate the individual user’s needs rather than requiring the user to adapt to the controller. The company also manufactures the Locking Shoulder Joint for shoulder disarticulation patients.
Below-the-ankle amputees can take comfort in the BiOM Ankle System from iWalk, which was founded by Hugh Herr, a double amputee and world-renowned mountain climber who lost both of his legs following a disastrous expedition on Mt. Washington in 1982. He is director of biomechatronics at MIT. With funding provided by the U.S. Department of Veterans Affairs and the Army’s Telemedicine and Advanced Technology Research Center (TATRC), the Powered Human Augmentation project was established and bionic technology was applied to develop the first lower-extremity device that powers movement and enhances mobility.
The BiOM Ankle System provides artificial muscle power that enables amputees to walk with the same gait and the same effort as non-amputees, reducing fatigue and pain, improving stability and balance, and improving overall quality of life. It uniquely features powered propulsion, stiffness modulation, and personal bionic tuning.
Also, the system restores normal ankle motion by providing a powered push-off that increases energy return by 100% and mechanical power delivery by 800%, compared to all other commercially available ankle-foot prosthetics. It can modulate ankle stiffness as well, improving gait and stability while enabling lower-limb amputees to smoothly decelerate the body after a heel strike just as they would on a biological ankle. Its personal bionic tuning system allows adjustments, via Bluetooth, so each individual’s gait falls within normal parameters for a non-amputee.
Drug Delivery And Management
The Raisin system from Proteus Biomedical optimizes therapeutic benefits for those on frequent and timely medications. Patients typically don’t take 30% to 50% of their prescribed medications, and the costs of hospitalization due to non-adherence are very high. The Raisin system marries medicine and mobile computing technologies to solve this problem.
This integrated pharmaceutical system identifies the pill, its authenticity, and its dosage when it is ingested. It then detects the pills and assigns time stamps. Also, it measures ECG signals, activity, and sleep and collects and presents data in real time. It then serves as a platform for data sharing, collaboration, and incentives.
The system consists of a handheld monitoring device, ingestible event markers (IEMs), and a receiver patch. The tiny marker chips, measuring less than 1 mm, have been tested as accurate at more than 99% in drug detection in multiple human clinical trials. It produces an ECG-like but non-RFID signal conducted only in the body.
Thin-film MEMS layers on each IEM are activated and powered using stomach electrolytes. The system modulates/pulses the current flow to encode information stored in it. It then communicates this data through the body tissue, where a receiver worn on the patient’s skin detects an electric field.
A departure from conventional planar MEMS manufacturing to high-aspect micromachining (HARM) processing has allowed Coto Technology Inc. to produce the RS-A-2515 magnetic RedRock reed switch (see “Micro-Fabricated Magnetic Reed Switch Sets Size And Performance Record”). It solves the size problem of conventional reed switches yet retains all of the high-performance properties of inherent classical magnetic reed switches.
The RedRock is the industry’s smallest micro-fabricated magnetic reed switch, with a footprint of less than 2.1 mm2 (1.01 by 2.08 mm) and a height of just 0.94 mm. These switches target ingestible endoscopic capsules, insulin delivery for implantable pumps, and hearing aids (Fig. 6).
This file type includes high resolution graphics and schematics.