Use Paper-Mill Biomass Waste for Lithium-Sulfur Battery Cathode
Repurposing a widely available “waste” material for a new use is a winning approach in terms of cost savings and environmental benefits. It’s now become an option with lignosulfonate, a sulfonated-carbon waste material that’s an abundant byproduct of the papermaking process. It’s typically burned on site, releasing CO2 into the atmosphere after sulfur has been captured for reuse.
However, a team based at Rensselaer Polytechnic Institute, Troy, N.Y., is taking it in another direction—they developed a technique for treating and then using this biomass to create part of rechargeable lithium-sulfur batteries.
“Our research demonstrates the potential of using industrial paper-mill byproducts to design sustainable, low-cost electrode materials for lithium-sulfur batteries,” said Trevor Simmons, the research scientist who developed the technology along with colleagues at the Center for Future Energy Systems (CFES). The details are in their paper “Repurposing paper by-product lignosulfonate as a sulfur donor/acceptor for high performance lithium–sulfur batteries,”published in the journal Sustainable Energy & Fuels, with further information in a supplemental package. Along with former graduate student Rahul Mukherjee, Simmons has patented the process, Patent #9,859,561.
In a lithium-sulfur battery, the cathode is composed of a sulfur-carbon matrix, and a lithium-metal oxide is used for the anode. While elemental sulfur is nonconductive, it can be made highly conductive by combining with carbon at elevated temperatures, thus making it suitable for battery technologies.
However, there’s a problem as the sulfur can dissolve into a battery’s electrolyte, which causes the cathode and anode electrodes to deteriorate after only a few cycles. To overcome this, batteries typically use carbon-based materials (including nanotubes) to confine the sulfur in its place.
1. The reuse process is based on processing of lignosulfonate, also called brown liquor, which is a waste byproduct of the paper-production process. (Source: Rensselaer Polytechnic Institute)
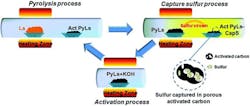
The Rensselaer researchers worked with a nearby paper mill, which provided the lignosulfonate, commonly referred to as “brown liquor” (Fig. 1). This dark, syrupy substance was dried and heated to about 700°C in a quartz-tube furnace. The intense heat drives off most of the sulfur gas but retains some of the sulfur as polysulfides (chains of sulfur atoms) embedded deep within an activated carbon matrix. The heating process is continued until the desired amount of sulfur is trapped in the carbon matrix (Fig. 2).
2. Shown is a schematic of the circulatory synthesis procedure of the activated pyrolytic lignosulfonate-captured sulfur (Act PyLs-Cap S) composite material. (Source: Rensselaer Polytechnic Institute)
Due to this repetitive pyrolysis process with carbon activation and sulfur capture, the carbon/sulfur composite material had a high proportion of surface area to material mass. The resultant material is the then ground up and mixed with an inert polymer binder to create a cathode coating on aluminum foil.
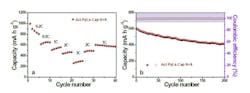
The team built button-size lithium-sulfur test batteries using the material as the cathode, and measured a capacity-decay rate over about 0.1% per cycle over 200 cycles, with capacity up to 1100 mA-hr/gm (Fig. 3).
3. Rate capability (a) and cycling performance and Coulombic efficiency (b) of the Li-S batteries with Act PyLs-Cap S+S electrode at high sulfur-content loading (~3.5 mg/cm2). (Source: Rensselaer Polytechnic Institute)
After 200 cycles, the reversible capacity remained around 410 mA-hr/gm, with a capacity decay rate of 0.18% per cycle. The team’s plan is to scale up the prototype to increase the discharge rate and the battery’s cycle life.
Initial funding for the research came from the New York State Pollution Prevention Institute (NYSP2I). The research team also obtained a Bench to Prototype grant from the New York State Energy Research and Development Authority, administered through NY-BEST (New York Battery and Energy Storage Technology), to more fully develop the technology.
About the Author

Bill Schweber
Contributing Editor
Bill Schweber is an electronics engineer who has written three textbooks on electronic communications systems, as well as hundreds of technical articles, opinion columns, and product features. In past roles, he worked as a technical website manager for multiple topic-specific sites for EE Times, as well as both the Executive Editor and Analog Editor at EDN.
At Analog Devices Inc., Bill was in marketing communications (public relations). As a result, he has been on both sides of the technical PR function, presenting company products, stories, and messages to the media and also as the recipient of these.
Prior to the MarCom role at Analog, Bill was associate editor of their respected technical journal and worked in their product marketing and applications engineering groups. Before those roles, he was at Instron Corp., doing hands-on analog- and power-circuit design and systems integration for materials-testing machine controls.
Bill has an MSEE (Univ. of Mass) and BSEE (Columbia Univ.), is a Registered Professional Engineer, and holds an Advanced Class amateur radio license. He has also planned, written, and presented online courses on a variety of engineering topics, including MOSFET basics, ADC selection, and driving LEDs.