Metal-Free Battery is Fully Biodegradable
What you'll learn:
- Truly organic, metal-free biodegradable batteries are possible.
- Their chemistry differs radically from conventional batteries.
- These batteries biodegrade into harmless amino acids.
Most conventional batteries are recyclable in theory regardless of their chemistry and materials, but in practice the majority of batteries are “tossed” and end up in landfills. Except for car batteries (for which solid numbers are available, with about 90% of their lead-acid batteries in the U.S. recycled), the numbers for other battery types are far, far lower, vary tremendously from country to country, and have large uncertainties. All of these trashed batteries add to waste mass and also represent a loss of irreplaceable material and minerals. That’s why a truly biodegradable battery would offer genuine advantages.
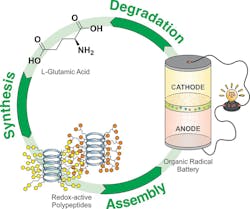
That’s the goal of a project carried out by team headed by Jodie Lutkenhaus, Professor of Chemical Engineering and Materials Science & Engineering at Texas A&M University. They developed a metal-free battery using an organic polypeptide “backbone” for its electrodes, which can be degraded with acid into amino acids for potential reuse. The amide bonds along the peptide backbone are stable during battery operation but can be triggered to break down for recycling (Fig. 1).
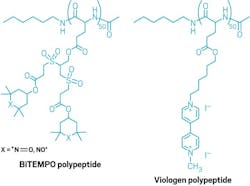
In basic chemical terms, they started with l-glutamic acid polypeptide chains, then attached 2,2,6,6-tetramethyl-4-piperidine-1-oxyl (biTEMPO) to make the cathode and 4,4′-bipyridine derivatives (viologen) to make the anode (Fig. 2). A radical-based chemical mechanism “shuttled” electrons between the two structures.
A battery is more than disparate elements; it must be assembled as well. Overall construction of this cell is similar to one using a lithium electrode (Fig. 3).
Of course, batteries also are about performance. The storage density of this battery, with a terminal voltage between 1.1 and 1.6 V, is about 38 mA-hr/gm and 60 mW-hr/gm, which about one-third that of lithium-based sources. Furthermore, according to their detailed, fully referenced comparison table, it’s in the mid-range of “organic” cells that are non-degradable.
To induce decomposition of the battery, the team added hydrochloric acid and then used a mass spectrometer to confirm that the battery compound disintegrated into l-glutamic acid, n-hexylamine, and other hydrolysis products. They note that even if these batteries don’t make it to a recycling center, they would still eventually break down naturally in the environment into these compounds.
The work was supported by the National Science Foundation, the Welch Foundation, and the U.S. Department of Energy Office of Science. It’s detailed in their paper in Nature with the surprisingly brief title “Polypeptide organic radical batteries,” along with a very detailed “Supplementary Information” packet. In addition, there’s an interesting, brief stage-by-stage recounting by Prof. Lutkenhaus, “The Path to Polypeptide Organic Radical Batteries,” in Device and Materials Engineering, describing how the team worked through the various problems they encountered as the project progressed. The article adds a real-world perspective not often seen in the formal research presentations.