Optogenetics probes offer insight to brain diseases
Optogenetics—the use of light to control living cells that have been genetically modified to be light-sensitive—could transform neuroscience if the technique could safely be applied to humans. The technology can help pave the way to a greater understanding of the brain and toward the development of novel treatments for brain disorders such as Alzheimer’s, schizophrenia, autism, and epilepsy. Optogenetics requires, first, the genetic engineering of neurons to make them light-sensitive. The second step involves getting light to the sensitized cells.
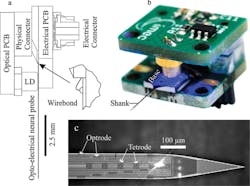
This second step was the focus of research described by imec, KU Leuven, and Neuro-Electronics Research Flanders in a paper1 presented at the 2015 IEEE International Electron Devices Meeting. The researchers presented a set of silicon neural probes that combines 12 monolithically integrated nanophotonic circuits, or optrodes (which optically stimulate single neurons), fabricated using a CMOS-compatible process. The fully integrated implantable probes enable optical stimulation and electronic detection of individual neurons.
The project builds on imec’s work with artificial synapses and brain stimulation and recording probes extending back to 2007. Dries Braeken, team leader for the project at imec, said in a phone interview, “The work adds functionality and complexity to what we have done before.” The brain is composed of many genetically and functionally distinct neuron types, and the new probes overcome the limitations of conventional probes, which cannot disambiguate recorded electrical signals with respect to their source. Braeken explained that optical stimulation can target very specific cell types vs. the more difficult-to-control electrical stimulation.
To build the probe, Braeken said the researchers integrated two CMOS processes: silicon nitride photonics and titanium nitride electrodes. The probes are 100 µm wide and 30 µm thick, containing 12 optrodes (6 x 20 µm2 in size) and 24 electrodes (10 x 10 µm2). The researchers packaged the circuitry, implanted it in a mouse brain, and successfully demonstrated that it could both drive and record neural activity (see the illustration).
Courtesy of imec/IEDM Editor Press Center
According to Braeken, it’s well-known that humans with Parkinson’s disease are treated using electrical stimulation; the optogenetic approach could offer a much more efficient and effective way of treating any neural disease. The technique, he said, also has been tested on cardiac cells. “There could be many applications for this technology,” he said.
Braeken said the imec and KU Leuven probes have been used experimentally on rodent brains, but he cited an article2 commenting on moving optogenetics technology to human applications. For example, Circuit Therapeutics wants to begin clinical trials using optogenetics to treat chronic pain. And RetroSense Therapeutics plans to soon begin human trials of optogenetics for treating a genetic condition that causes blindness.
The development of probe technology is only one aspect of exploiting the promise of optogenetics. Edward S. Boyden, an MIT neuroscientist, found that his neural probing of a mouse brain was generating more data—a terabit per second—than his computers could handle.3 Boyden worked with startup LeafLabs on the problem, leading to the development of a data-acquisition system called Willow for neuroscience applications. A Willow module communicates concurrently with as many as 32 industry-standard neural amplifier chips, and it contains an FPGA that processes 1,000+ channels of electrophysiological data. The FPGA writes data directly to a storage drive while simultaneously forwarding the data to a computer for real-time monitoring and feedback.
The application of the computer is described in the paper4 by Boyden and several coauthors: “Here we present a novel architecture in which a digital processor receives data from an analog-to-digital converter and writes that data directly to hard drives, without the need for a personal computer to serve as an intermediary in the DAQ process.” This minimalist architecture, they write, will facilitate the future scaling of electrophysiological recording.
References
- Hoffman, L., et al., “High Density Optrode-electrode Neural Probe using SixNy Photonics for In Vivo Optogenetics,” Session 29, IEDM, December 2015.
- Sutherland, S., “Revolutionary Neuroscience Technique Slated for Human Clinical Trials,” Scientific American, Jan. 5, 2016.
- Carpenter, M., “Glut of data from mice brains tests MIT’s computing power,” The Boston Globe, Jan. 31, 2016.
- Kinney, J. P., et al., “A direct-to-drive neural data acquisition system,” Frontiers in Neural Circuits, Sept. 1, 2015.