Electronics and related technologies play key roles in medical applications. Those roles include acquiring data, processing it, communicating it to caregivers, allowing caregivers to collaborate (often remotely), and administering some form of treatment.
While diagnostic data can come from a variety of traditional tools like EKG, PET, MRI, and ultrasound systems, data is increasingly coming from portable devices that can be used outside a clinical setting. Whatever the source, test is an important part of the process of making sure sensors and systems are functioning properly.
Sensors
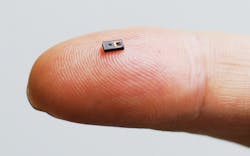
Several sensor products are coming to market for medical and healthcare applications. Kyocera, for example, makes a variety of electronic components for applications ranging from smartphones to automotive driving/safety equipment and infotainment systems. The company said that as part of an initiative to build slimmer, smaller products to support higher functionality in more compact devices, it has developed what it calls one of the smallest known optical blood-flow sensors (Figure 1) that measures the volume of blood flow in subcutaneous tissue. Kyocera said the device could find use in a variety of mobile health (mHealth) applications—such as monitoring stress levels or preventing dehydration, heatstroke, and altitude sickness by monitoring changes in blood-flow volume and analyzing the changes with new algorithms to detect specific conditions.
Courtesy of Kyocera
The wearable device market has expanded substantially in recent years, focused primarily on health and fitness. Kyocera cited information issued by Tractica LLC in April 2016 predicting that global shipments of healthcare wearables are expected to rise from 2.5 million units in 2016 to 97.6 million units in 2021, driven by new mHealth applications including the need to treat chronic diseases, provide eldercare, and maintain wellness.
The company said the new sensor—1 mm high, 1.6 mm long, and 3.2 mm wide—leverages Kyocera’s expertise in miniaturization, enabling it to find use in mobile phones and wearable devices. The company developed this sensor as an integrated module, incorporating a laser diode and photodiode into a single ceramic package. When light is reflected from blood cells within a blood vessel, the frequency of light varies in accordance with a Doppler shift according to the blood-flow velocity. The new sensor utilizes the Doppler shift in frequency (which increases as blood flow accelerates) and the strength of the reflected light (which grows stronger when reflected off a greater volume of red blood cells) to measure blood-flow volume.
Devices equipped with this new sensor will be able to measure blood-flow volume in subcutaneous tissue by placing the device in contact with an ear, finger, or forehead. (The sensor targets capillaries for measurement and cannot be utilized on all parts of the body.) Featuring a high signal-to-noise ratio, small size, and low power consumption (0.5 mW), the sensor can be integrated into a smartphone or wearable device for mHealth applications.
The company will offer sensor-module samples starting in April and aims to commercialize the technology by March 2018.
Biometric wearables
In other sensor news, Valencell, a developer of performance biometric data sensor technology, and STMicroelectronics announced in December the launch of a scalable development kit for biometric wearables that includes ST’s compact SensorTile turnkey multisensory module (see “Evaluation kits, reference designs offer a head start,” page 18) integrated with Valencell’s Benchmark biometric sensor system. The companies said integrating ST’s SensorTile development kit with Valencell’s Benchmark sensor technology simplifies the prototyping, evaluation, and development of wearable and IoT solutions by delivering a complete Valencell PerformTek technology package.
Valencell said Benchmark incorporates Valencell PerformTek biometric sensor technology in a prepackaged system for immediate integration in wearables. Benchmark is available in two versions:
- for biometric wrist/arm wearables and
- for biometric earbud hearables.
Valencell said its PerformTek sensor systems provide accurate, robust, and flexible technology, giving wearable and hearable devices the capability to continuously and accurately measure blood-flow signals, even during extreme physical activity or when the optical signals are weak. These signals can be translated into biometric data, including continuous heart rate, VO2 and VO2 max, resting heart rate, heart-rate response, heart-rate recovery, continuous-energy expenditure (calorie burn), cardiac efficiency, and heart-rate variability assessments.
“Working with ST has allowed us to bring together the best of all sensors required to support the most advanced wearable use cases through our Benchmark sensor system,” said Dr. Steven LeBoeuf, president and cofounder of Valencell, in a press release. “What attracted us to the SensorTile was the flexibility of the platform and the ultralow power consumption, which will enable our customers to create highly accurate and powerful wearables and hearables in any form factor.”
Test and measurement
A key consideration for products based on sensors like Kyocera’s blood-flow sensors or Valencell’s Benchmark PerformTek sensors is power consumption. The sensors may have impressive data-sheet specs, but it remains necessary to make sure they offer extended battery life in the final product as it will be used by the customers.
To that end, Keysight Technologies offers oscilloscopes, DMMs, DC power analyzers, and low-current analyzers for medical and healthcare test. Hong-Kee Fong, medical and education solutions manager at the company, said a key focus for medical and related applications is battery life maximization for smart devices and sensors.
He explained by way of background that the IoT simplifies and enables remote monitoring, with a key component of remote monitoring being the capability and reliability of the sensors. “Another key requirement of all these remote sensors is battery life,” he added. “A sensor is only as good as the battery.”
He continued, “A key factor in all these IoT sensors is the need to last a long time, and in order for them to do this, they must have an aggressive power-management strategy that draws as little current as possible when they sleep, and they may draw high currents when operational (this often ranges from nA to A). [Measurement] products should have a dynamic range that can cover these requirements seamlessly. The other power-management strategy is to pulse the current drawn. And these devices vary the frequency of this drawn current.” Consequently, he added, instruments need to have the bandwidth capable of capturing these frequency variations.
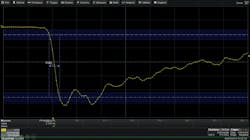
Fong cited the Keysight CX3300 Series device current waveform analyzer (Figure 2) as an instrument suited for researchers struggling with high-speed transient current measurements during advanced device characterization and for engineers working to reduce power/current consumption in low-power devices. The CX3300 analyzer enables the simultaneous measurement of wideband and low-level current waveforms. By providing a wide dynamic measurement range, a single CX3300 analyzer can meet a range of measurement requirements without using multiple instruments.
Courtesy of Keysight Technologies
The CX3300 analyzer consists of a mainframe with a WXGA 14.1-inch display and current sensors specifically dedicated to precision current waveform measurements. The mainframe features a 14-bit or 16-bit wide dynamic range, 200 MHz of maximum bandwidth, and a sampling rate of 1 GS/s. The low noise and wideband current sensors support dynamic current measurements from 10 A down to 100 pA level.
High-definition oscilloscopes
Mike Hertz, field applications engineer at Teledyne LeCroy, said his company’s oscilloscopes can be used to acquire medical electronics signals in a wide range of medical applications, involving EKG, PET scans, MRI, ultrasound, and more.
A tech brief from the company illustrates some of the challenges involved. “When testing electronics in a lab, an engineer can inject a known test signal that is always the same shape and then look at the output of the device under test.”1 With signals generated by humans, however, “There is no repetitive signal source: Each heart beat is a bit different, and each firing of an electrical synapse differs from the previous ones. Often the signal-to-noise ratio is not nearly as good as when testing other types of signals. And yet, a high degree of accuracy is needed. From an engineering point of view, the test equipment needs to make good measurements of ‘single-shot’ signals (no averaging because the signal is not repetitive) in conditions where the noise level is significant.”
For medical test applications, Hertz pointed to Teledyne LecCroy’s family of true 12-bit vertical-resolution High Definition Oscilloscopes with advanced measurement and analysis capabilities. He provided by way of example a screenshot showing measurements of a PET scan waveform from a photomultiplier tube (Figure 3). Other oscilloscope applications include oxygen displacement measurement and analysis of ultrasound signals to detect cancerous tissue and blood clots.
Courtesy of Teledyne LeCroy
Hearing-aid test
Averna also offers test systems for medical devices. For example, the company reported that it developed flexible test equipment for the implantable part of its customer’s implantable hearing-aid system. Averna provided the hardware and software test infrastructure required to completely validate the customer’s PCB-mounted IC that sends electronic signals to the implantable actuator directly stimulating the cochlea.
Averna employed a multidisciplinary approach—combining electronic, mechanical, and software solutions. “After conducting an interactive kick-off meeting and comprehensive preliminary studies, we were able to design and produce modular test equipment,” explained Averna project engineer Kurt Aerts. “Throughout this process, we documented all the necessary product specifications, requirements, and results in the most detailed manner. That way, our client was able to maintain a clear overview of the effectiveness of our solution at all times, while conforming to strict documentation requirements essential in the medical field.”2
Averna said it produced three automated systems for the customer. Added project engineer Tom Oyen, “From basic functionality tests for electronic components, for instance, to very specific distortion tests that verify the quality of the auditory signals transmitted, our solution enables our client to test hundreds of all-important features related to the quality of its implantable hearing aids.” The test systems employed PXI instruments as well as the NI LabVIEW graphical programming environment and NI TestStand test-management software.
Astronics offers integrated test solutions for medical electronics test and other applications for customers who find that “one-size-fits-all” testers are far from ideal. The company says it draws on “a broad base of technologies and systems components” to deliver an effective solution quickly.3
Astronics will handle test-system design, including hardware and software integration, manufacturing and supply-chain management, design verification and acceptance testing, on-site installation and calibration, and ongoing support, including maintenance, repair, and training. The company cites a portable defibrillator test system as an example of its capabilities in the medical area.
Detector monitoring
In addition to enabling biomedical sensors and the test of sensors and medical-electronics devices and systems, electronic technology has other roles to play—for example, enabling electronic health records,4 facilitating physical therapy,5 or monitoring equipment performance issues.
In this last category, Konica Minolta Healthcare at the November 2016 Annual Meeting of the Radiological Society of North America (RSNA) in Chicago introduced the AeroRemote monitoring service for Konica Minolta AeroDR digital radiographer detectors. The company described AeroRemote as a cloud-based, active monitoring solution that detects and reports critical and ongoing performance issues with the AeroDR flat-panel detectors, providing automatic reports that can be reviewed anywhere, anytime.
AeroRemote provides the capability to address potential system or user issues early, before they escalate. It offers secure, real-time information needed to maximize system utilization, minimize service interruptions, and evaluate personnel performance. It produces monthly reports, by system and user, detailing usage and personnel productivity, providing at-a-glance information that facilitates a more efficient operation, supports user best practices, and optimizes AeroDR imaging assets.
Using predictive analytics, AeroRemote gathers hardware status to avoid downtime by scheduling service before problems occur. Real-time monitoring of system parameters and events—from drop detection to component performance—lets users make faster and better-informed support decisions. In addition, it encourages proper user handling by alerting managers when a digital radiography (DR) panel has been dropped and sends diagnostic information on panel performance using the panel check tool.
“AeroRemote reflects the transformation of service that’s underway,” explained Steven Eisner, senior product marketing manager, Konica Minolta Healthcare Americas, in a press release. “It represents an important step in proactive management by providing more data to make better service and usage decisions that can reveal a lot of information, improving productivity and maximizing patient safety. For users of our AeroDR Systems, this moves the support needle from reactive and preventive to predictive and prescriptive management.”
Also at RSNA, Konica Minolta Healthcare introduced its next-generation AeroDR HD digital radiography detector, which combines high sensitivity and smaller pixel size in a lightweight and waterproof enclosure. The high-definition flat-panel detector delivers a 100-micron pixel size for a 3,488 x 4,256 pixel count—four times more than standard resolution.
“The AeroDR HD Detector utilizes so many more pixels per image that specialists are able to see areas of interest in exquisite detail for better, faster patient care decisions,” said Guillermo Sander, senior strategic marketing manager of digital radiology at Konica Minolta Healthcare Americas. “The technology provides subtle, but critical, detail with dose efficiency in a design that improves workflow and image acquisition.”
The AeroDR HD measures 14 inches x 17 inches and weighs 5.7 pounds. Designed for portability with a protective waterproof enclosure and a new ergonomic design, it absorbs impacts from bumps and drops. It achieves crisp, clear visualization in a variety of settings, yet the technology is still dose-efficient to patients, the company said. The new AeroDR HD Detector is compatible with the family of other AeroDR detectors.
Collaboration
Calgary Scientific, a provider of innovative solutions that empower clinicians by enabling collaborative and secure access to applications from web and mobile devices, chose RSNA to announce version 6.0 of its ResolutionMD enterprise image viewing platform. The goal is to expand interoperability across the healthcare enterprise to offer institutions a better way to connect clinicians and patients to each other and their data by providing a scalable pathway and seamless integration among cloud archives, EMRs, patient portals, and hospital-developed apps.
The new version includes multimonitor support, expanded DICOM (Digital Imaging and Communications in Medicine) modalities in departments outside radiology, extended image sharing with third-party providers, FHIR (Fast Healthcare Interoperability Resources) data integration, expanded HTML5 capabilities, and mobile measurements. Scalability improvements incorporate both virtualized and physical server deployments with CPU or GPU options.
“Interconnecting IT systems with the ResolutionMD platform means that physicians can securely access any data from inside or outside of the hospital enterprise from web and mobile devices,” said James Henry, CTO/EVP at Calgary Scientific, in a press release. “Uncompromised access along with the ability to start real-time multiparty collaborations provides critical information so practitioners can provide the best possible care.”
Healthcare in rural areas
ResolutionMD is a collaborative tool dedicated to medical applications. In contrast, Avaya’s Scopia videoconferencing product has found use in organizations ranging from ticketing and music production companies to government agencies.
As an example of a medical application, Avaya applied Scopia to facilitate emergency patient care in rural Germany. The company said that for St. Josef’s Hospital Weisbaden GmbH, Avaya Scopia videoconferencing helps coordinate care teams and specialists across the hospital’s three locations (Figure 4).
Courtesy of Avaya
Hans-Jürgen Jobst, senior product marketing manager, Avaya Germany, said the Silicon Valley-based company has customers in more than 60 countries and has established a longstanding history of business in the healthcare market in Germany and Europe. For its healthcare customer base in Germany, he said, Avaya provides “… mainly communications technology but also networking and emerging video technology.”
He commented on the challenges of serving rural areas. “In Germany, our towns are closer together than in the rural United States, but we have the situation where more and more people are moving to larger cities, meaning some smaller hospitals are losing experts and finding it challenging to operate,” he said. “Furthermore, special rural areas are characterized by an increasing shortage of doctors and an increasing number of patients.”
That situation pertained in the Rheingau region of Germany, he said, with one big hospital in Wiesbaden and then two peripheral hospitals in smaller towns. “The challenge was to overcome the ‘brain-drain’ and provide the same medical competency to the more remote sites that was available at the larger hospital in Wiesbaden; this is done via the use of video technology.”
He noted that videoconferencing can facilitate daily meetings in which specialists and experts at the main site can provide advice and guidance to the doctors in the peripheral sites. “Going further,” he said, “videoconferencing can connect doctors outside of hospital-to-hospital arrangements to more distributed doctors and individual clinics”—further projecting expertise to areas that wouldn’t normally have access to specialists and the services they provide.
When asked about connectivity speeds in rural areas, he noted that the smaller hospitals did upgrade their WAN connections. However, even for small towns where connecting via DSL is not an option, he said, “You can run the Scopia solution via a 200- to 300-kb/s connection to a patient using 3G or LTE connectivity, which means you also can provide video communication between doctors and people at home.”
Jobst commented on other issues as well. “In Germany, the new E-Health laws lay the groundwork for the digitalization of healthcare and make it possible to do some treatments via videoconference,” he said. “This has been an important cornerstone for enabling the introduction of video into healthcare.”
He continued, “Aside from a country’s legal framework, implementing video into healthcare takes a lot of buy-in from various people within the specific healthcare facility”—such as the CEO or head of the hospital or healthcare provider, the doctors on the ground who will actually work with this technology, and the IT department, which is tasked with the rollout and maintenance of the technology.
Beware technology overuse
From sensors to connectivity, healthcare innovations will continue to roll out. But some researchers caution against overuse of technology. Consider consumer-use baby monitors. “These devices are marketed aggressively to parents of healthy babies, promising peace of mind about their child’s cardiorespiratory health,” said pediatrician and safety expert Christopher P. Bonafide, M.D., MSCE, of Children’s Hospital of Philadelphia (CHOP). “But there is no evidence that these consumer infant physiological monitors are life-saving or even accurate, and these products may cause unnecessary fear, uncertainty, and self-doubt in parents.”6
Bonafide, along with Elizabeth E. Foglia, M.D., MSCE, a neonatologist at CHOP, and David T. Jamison, executive director of Health Devices at ECRI Institute, a nonprofit research organization, wrote an article in the Journal of the American Medical Association7 addressing the issue.
“There is no publicly available evidence that these baby monitors are accurate in measuring a baby’s vital signs,” said Jamison. “And since these baby monitors are not regulated by the FDA, we have to question what testing has been done to assure the safety and quality of these designs.”
Added Foglia, “In the future, some physiological monitors may offer real benefits to vulnerable infants at home, but we have no evidence now that these devices are safe, accurate, or effective.”
References
- Lauterbach, M., “Testing Medical Electronics,” Teledyne LeCroy, Technical Brief, Aug. 15, 2012.
- “Averna delivers a 100% sound solution for hearing implants,” Averna, Case Study.
- Test System Solutions, Astronics Test Systems, Brochure, 2016.
- Lecklider, T., “More data than you can imagine,” EE-Evaluation Engineering, May 2015, p. 20.
- Nelson, R., “Robotics augments stroke rehabilitation,” EE-Evaluation Engineering, February 2017, p. 28.
- “Consumer-Use Baby Monitors Have Little Proven Benefit for Healthy Infants,” Newswise, Jan. 24, 2017.
- Bonafide, Christopher P., et al., “The Emerging Market of Smartphone-Integrated Infant Physiological Monitors,” Journal of the American Medical Association, Jan. 24-31, 2017.
For more information
About the Author

Rick Nelson
Contributing Editor
Rick is currently Contributing Technical Editor. He was Executive Editor for EE in 2011-2018. Previously he served on several publications, including EDN and Vision Systems Design, and has received awards for signed editorials from the American Society of Business Publication Editors. He began as a design engineer at General Electric and Litton Industries and earned a BSEE degree from Penn State.