Can Nano-Engineered Anodes Boost Li-ion Battery Capacity and Longevity?
What you’ll learn:
- A “hybrid” anode material shows potential to improve the capacity and longevity of lithium-ion batteries.
- An exclusive interview with Electronic Design reveals details about the material, developed at Korea’s Dongguk University, which combines reduced graphene oxide with nickel-iron layered double hydroxides to create a highly conductive structure that offers high capacity and supports rapid charging.
- Learn more about the unique nano-assembly process that produces the hollow “hybrid” structures and how it can be integrated with existing Li-ion manufacturing processes.
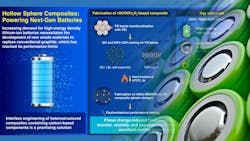
Researchers from South Korea’s Dongguk University recently released preliminary details on a novel hybrid anode material they describe as “a significant breakthrough in lithium-ion battery technology.” It could improve the performance, capacity, and longevity of cells produced in existing manufacturing facilities. The new anode material achieves these improvements with a nano-engineered structure that integrates graphene oxide's superior conductivity with the energy storage capabilities of nickel-iron compounds.
Their study, published in Chemical Engineering Journal,1 describes a hierarchical heterostructure composite that optimizes material interfaces at the nanoscale level (Fig. 1). It produces significant improvements in energy storage capacity and long-term cycling stability over cells using conventional anode materials.
Anode Composite’s Test Results Look Promising
Electrochemical tests conducted during the study revealed the anode material's exceptional performance. Specific capacity was 1687.6 mAh g−1 at a current density of 100 mA g−1 after 580 cycles, surpassing most conventional materials in capacity while exhibiting excellent cycling stability. In addition, the material exhibited good rate performance, maintaining high capacity even at significantly increased charge/discharge rates with a high charge/discharge capability (~283 mAh/g at 20,000 mA/g).
Researchers, led by Professor Jae-Min Oh of Dongguk University, in collaboration with Seung-Min Paek of Kyungpook National University, are addressing these challenges by engineering materials at the nanoscale. Their work focuses on a novel hybrid material designed to maximize the synergistic effects of its components.
This innovative composite is a hierarchical heterostructure that combines reduced graphene oxide (rGO) with nickel-iron layered double hydroxides (NiFe-LDH). This unique composite leverages the properties of its components: rGO provides a conductive network for electron transport, and the nickel-iron-oxide components enable fast-charge storage through a pseudocapacitive mechanism. The key to this innovative design is the abundance of grain boundaries, which facilitates efficient charge storage.
Professor Seung-Min Paek emphasized the collaborative nature of the research: “This breakthrough was made possible through close cooperation between experts in diverse materials. By combining our strengths, we were able to design and optimize this hybrid system more effectively."
Nano-Manufacturing a Hybrid Anode Structure
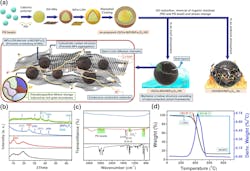
The researchers produced the composite material by means of a layer-by-layer self-assembly technique using polystyrene (PS) bead templates. First, the PS beads were coated with GO and NiFe-LDH precursors. The templates were then removed, leaving behind a hollow sphere architecture.
Following that, a controlled thermal treatment induced a phase transformation in NiFe-LDH. This led to the formation of nanocrystalline nickel-iron oxide (NiFe2O4) and amorphous nickel oxide (a-NiO), while simultaneously reducing GO to rGO (Fig. 2).
This synthesis resulted in a well-integrated hybrid composite (rGO/NiFe2O4/a-NiO) with enhanced conductivity, making it an efficient anode material for lithium-ion batteries. The hollow structure prevents direct contact between the a-NiO/NiFe2O4 nanoparticles and the electrolyte, improving stability. The team then used advanced characterization techniques, such as X-ray diffraction and transmission electron microscopy, to confirm the composite's formation.
The “Shocking Details”
In an exclusive interview with Electronic Design, Professor Jae-Min Oh provided additional details regarding the novel anode material’s potential for commercialization:
ED: What are the challenges involved with scaling production of the anode material up to commercial volumes?
Jae Min Oh: Scaling up the production of our rGO/a-NiO/ NiFe2O4-HS hybrid material for anode involved several challenges. First, the layer-by-layer self-assembly process using polystyrene templates is precise but may require optimization for throughput and cost at industrial scales.
Additionally, the heat-treatment step under inert atmosphere is essential to retain the hollow structure and reduce structural defects, which could increase production complexity and cost. Ensuring uniform particle size distribution and structural integrity during mass production will also be key to maintaining performance consistency.
That said, this study is a fundamental investigation focused on nano-fabrication strategies to enhance anode performance, and large-scale production was not the primary objective at this stage. However, as with many fundamental studies that later achieve commercialization, we believe that scale-up could be realistically achieved by applying chemical engineering techniques to control key process parameters.
Can existing Li-ion manufacturing facilities be adapted to use the new anode material or does it require an all-new manufacturing process? If they can be retrofitted, how much new equipment is required?
The proposed anode material is compatible with standard slurry-casting and electrode fabrication techniques used in current LIB manufacturing. However, upstream processes, such as the synthesis of hollow graphene/LDH hybrid spheres and controlled thermal treatment under inert gas, would likely require adaptation or additional modules. That said, once the active material is synthesized, its integration into conventional LIB architectures should not demand major retrofitting.
Will the new material add significant unit cost to the product? How will it change the cost per kWh?
As this study is focused on developing advanced materials to improve anode performance, we did not conduct a cost analysis. Factors such as raw material prices, processing scalability, and equipment requirements all contribute to the final production cost, making it difficult to provide a meaningful estimate at this early stage. Similarly, the cost per kWh would depend on many variables beyond the scope of this fundamental research.
Is there a rough estimate of the improvements in capacity, charge speed, and product lifetime that the new materials will offer when they are first introduced, and once the technology matures?
As we reported earlier, laboratory-scale tests showed that our anode material demonstrated a specific capacity of up to 1687.6 mAh/g after 580 cycles. We also learned that they demonstrated a high rate capability (sustaining ~283 mAh/g at 20,000 mA/g). This represents a significant improvement over conventional anode materials.
While it's too early to predict exact figures for commercial systems, the pseudocapacitive behavior and structural stability suggest promising potential for faster charge rates and longer lifespans as the technology matures and fabrication is refined.
What Lies Ahead?
Professor Jae-Min Oh summed up the achievement by saying, "We anticipate that, in the near future, energy storage materials will move beyond simply improving individual components. Instead, they will involve multiple interacting materials that create synergy, resulting in more efficient and reliable energy storage devices. This research offers a pathway to smaller, lighter, and more efficient energy storage for next-generation electronic devices."
Reference
1. Jae-Min Oh, Seung-Min Paek, “Phase change-induced heterointerface engineering of hollow sphere structured graphene oxide/layered double hydroxide composites for superior pseudocapacitive energy storage in lithium-ion batteries,” Chemical Engineering Journal, January 15, 2025, DOI: 10.1016/j.cej.2025.159671.
Next in This Edition of PowerBites
More PowerBites
About the Author
Lee Goldberg
Contributing Editor
Lee Goldberg is a self-identified “Recovering Engineer,” Maker/Hacker, Green-Tech Maven, Aviator, Gadfly, and Geek Dad. He spent the first 18 years of his career helping design microprocessors, embedded systems, renewable energy applications, and the occasional interplanetary spacecraft. After trading his ‘scope and soldering iron for a keyboard and a second career as a tech journalist, he’s spent the next two decades at several print and online engineering publications.
Lee’s current focus is power electronics, especially the technologies involved with energy efficiency, energy management, and renewable energy. This dovetails with his coverage of sustainable technologies and various environmental and social issues within the engineering community that he began in 1996. Lee also covers 3D printers, open-source hardware, and other Maker/Hacker technologies.
Lee holds a BSEE in Electrical Engineering from Thomas Edison College, and participated in a colloquium on technology, society, and the environment at Goddard College’s Institute for Social Ecology. His book, “Green Electronics/Green Bottom Line - A Commonsense Guide To Environmentally Responsible Engineering and Management,” was published by Newnes Press.
Lee, his wife Catherine, and his daughter Anwyn currently reside in the outskirts of Princeton N.J., where they masquerade as a typical suburban family.
Lee also writes the regular PowerBites series.